【研究意义】猪繁殖与呼吸综合征(Porcine Reproductive and Respiratory Syndrome,PRRS)是由猪繁殖与呼吸综合征病毒(PRRSV)引起的一种高度传染性疾病,临床上以母猪发热、厌食、早产、流产,产木乃伊胎、死胎、弱仔等繁殖障碍,及各种年龄猪呼吸困难和仔猪高死亡率为特征[1-6]。自20 世纪90年代以来,PRRS给世界养猪业造成了巨大的经济损失,严重阻碍了世界养猪业的发展。因此,研究疫苗免疫效果对PRRS的防控具有重要意义。
【前人研究进展】1996年我国报道了首例PRRSV。PRRSV不断演变,目前流行的PRRSV存在多样性和广泛重组现象,不同地方(区域)的流行毒株也存在多样性,使得现有疫苗的保护力不足,增加了该病的防控难度[7-9]。疫苗免疫是传染病防控的有效手段之一,PRRS疫苗分为灭活苗(KV)和弱毒苗(MLV)两类。PRRSV-MLV疫苗能够诱导保护性免疫反应[10-14],但在安全性和对异源毒株交叉保护方面令人担忧[15-16];PRRS-KV疫苗具有安全、不存在散布病毒和造成PRRS新疫源的危险、便于贮存和运输、对母源抗体的干扰作用不敏感等优点,但保护效果不确切[17-19]。养殖场疫苗选择多样,免疫程序也存在较大差异,导致免疫效果不佳,为PRRS防控工作带来更大的风险与挑战。
【本研究切入点】本研究在田间条件下采用4种PRRSV疫苗组合免疫仔猪,通过免疫日龄和不同疫苗类别的免疫方案,统计和分析PRRSV疫苗免疫效果,以期为养殖场提供和制定合理的免疫程序。【拟解决的关键问题】通过免疫后的抗体反应、病毒血症和生产参数评估PRRS疫苗免疫效果,筛选出PRRS疫苗最佳免疫方案,为PRRS防控提供参考。
1 材料与方法
1.1 试验材料
1.1.1 试验猪场概况 A、B、C 3个试验猪场,规模分别为760、868、835头基础母猪。3个养殖场保育仔猪在30~50日龄时表现喘气、呼吸困难、体温升高、咳嗽、消瘦、毛松,保育仔猪发病率分别为100%、20%和30%。采集部分发病仔猪肺、淋巴结,RT-PCR方法检测PRRSV核酸为阳性。3个猪场的疫苗免疫和发病情况见表1。
1.1.2 试验动物 选取 A、B、C 3个PRRSV阳性场,每个场200头仔猪,14日龄首免前采血检测,PRRSV核酸均为阴性。
表1 试验猪场保育仔猪疫苗免疫和发病情况
Table 1 Vaccine immunization and morbidity in piglets
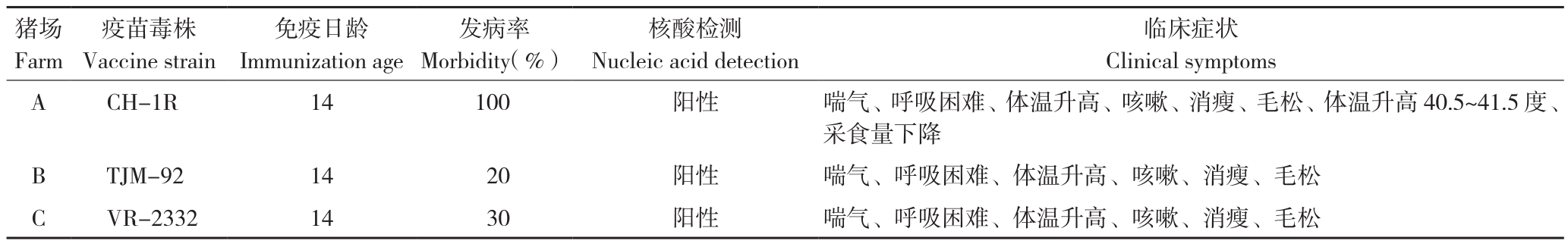
猪场Farm临床症状Clinical symptoms A CH-1R 14 100 阳性 喘气、呼吸困难、体温升高、咳嗽、消瘦、毛松、体温升高40.5~41.5度、采食量下降B TJM-92 14 20 阳性 喘气、呼吸困难、体温升高、咳嗽、消瘦、毛松C VR-2332 14 30 阳性 喘气、呼吸困难、体温升高、咳嗽、消瘦、毛松疫苗毒株Vaccine strain免疫日龄Immunization age发病率Morbidity(%)核酸检测Nucleic acid detection
1.1.3 试验疫苗 A、B、C 3个猪场分别采用CH-1R株、TJM-92株、VR-2332株不同PRRSV弱毒疫苗和HP-PRRSV灭活疫苗(NVDC-JXA1株),供试PRRSV疫苗均购自广州市生牧生物科技有限公司,疫苗信息如表2所示。
表2 供试PRRSV疫苗信息
Table 2 Information of PRRSV vaccines for trials
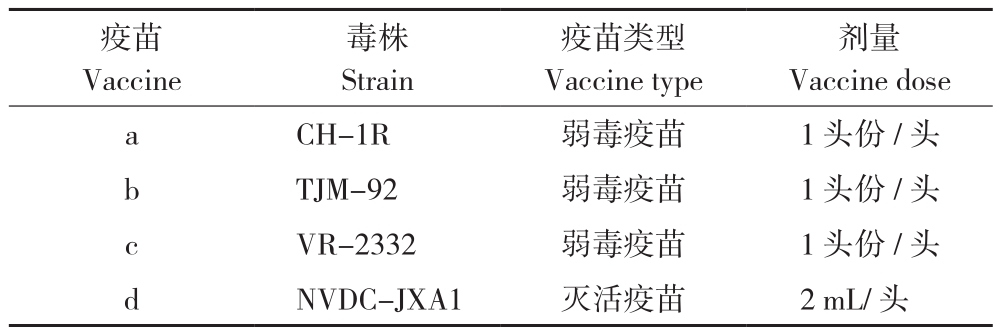
疫苗Vaccine剂量Vaccine dose a CH-1R 弱毒疫苗 1头份/头b TJM-92 弱毒疫苗 1头份/头c VR-2332 弱毒疫苗 1头份/头d NVDC-JXA1 灭活疫苗 2 mL/头毒株Strain疫苗类型Vaccine type
1.1.4 主要试剂 Rapure Viral RNA/DNA Kits 购自广州美基生物科技有限公司;一步法RT-PCR试剂盒、DL2000 DNA Marker购自宝生物工程(大连)有限公司;RPMI-1640培养基购自广州英伟创津有限公司;灭菌24孔细胞培养板购自广州市华粤行仪器有限公司;猪繁殖与呼吸综合征病毒抗体检测试剂盒(HerdChekPRRSX3, IDEXX Laboratories Inc., West brook, ME, USA)购自美国爱德士生物科技公司。
1.2 试验方法
1.2.1 猪群分组及免疫 3个PRRSV阳性场,每个养殖场200头仔猪,随机分成4组,每组50头。按照免疫方案,第1组于14日龄同时免疫弱毒苗MLV+灭活KV(包括A1、B1、C1);第2组于14日龄免疫MLV+35日龄免疫KV(包括A2、B2、C2);第3组于14日龄、35日龄免疫MLV(包括A3、B3、C3);第4组于14日龄免疫MLV(包括A4、B4、C4)。试验动物分组及疫苗免疫方案如表3所示。
表3 试验动物分组及疫苗免疫方案
Table 3 Immune program and experimental animal groups
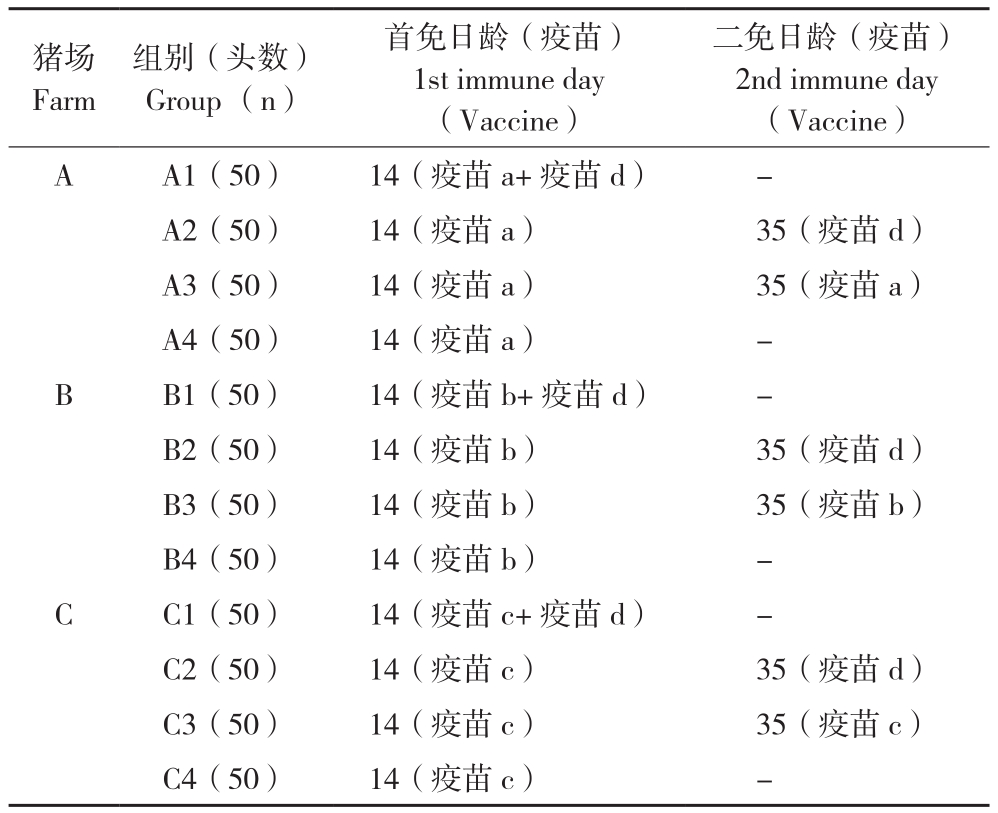
注:-表示未免疫。
Note: -represents no immune.
猪场Farm组别(头数)Group (n)首免日龄(疫苗)1st immune day(Vaccine)二免日龄(疫苗)2nd immune day(Vaccine)A A1(50) 14(疫苗a+疫苗d) -A2(50) 14(疫苗a) 35(疫苗d)A3(50) 14(疫苗a) 35(疫苗a)A4(50) 14(疫苗a) -B B1(50) 14(疫苗b+疫苗d) -B2(50) 14(疫苗b) 35(疫苗d)B3(50) 14(疫苗b) 35(疫苗b)B4(50) 14(疫苗b) -C C1(50) 14(疫苗c+疫苗d) -C2(50) 14(疫苗c) 35(疫苗d)C3(50) 14(疫苗c) 35(疫苗c)C4(50) 14(疫苗c) -
1.2.2 抗体检测 免疫前0 d(14日龄)、二免后30 d(65日龄)对仔猪经前腔静脉采血,编号,3 000 r/min离心10 min分离血清,-20℃保存备用。按照PRRSV抗体ELISA试剂盒的操作说明书对待检血清进行检测。
1.2.3 血清中PRRSV核酸检测 利用RT-PCR检测方法对血清中PRRSV核酸进行检测。根据Rapure Viral RNA/DNA Kits试剂盒说明提取核酸。一步法RT-PCR反应体系(50 μL):PrimeScript 1step Enzyme Mix 4 μL,上游引物 1 μL,下游引物1 μL,模板4 μL,Buffer 25 μL,无RNA酶H2O 15 μL 。反应程序:50℃反转录反应30 min;72℃ 1 min;94℃预变性 2 min、94℃ 50 s、54℃ 1 min,35个循环;72℃延伸10 min;4℃保存。反应结束后取10 μL RT-PCR产物用1.5%琼脂糖凝胶(含0.5 μg/ mL溴化乙锭)电脉,最后用紫外凝胶成像系统观察、拍照。
1.2.4 生产参数统计分析 统计试验过程中患有呼吸疾病仔猪头数、仔猪发病率和仔猪死淘率3个生产参数,对比分析各组合免疫效果。利用Graph Pad Prism 5软件中的Unpaired Student’s t-test方法进行差异显著性分析。
2 结果与分析
2.1 免疫PRRS疫苗前后抗体检测结果
从表4可以看出,免疫前0 d(14日龄)抗体视为母源抗体。A、B、C养殖场免疫前0 d(14日龄)的平均抗体水平S/P值分别为1.4、0.995和1.32。二免后30 d(65日龄)仔猪抗体检测结果显示,A养殖场A2组抗体最高,S/P值达到2.5,但与A1、A3、A4抗体水平无显著差异;B养殖场:B3组抗体最高,S/P值达到2.63,但与B1、B2、B4抗体水平无显著差异;C养殖场C3组抗体最高,S/P值达到2.99,但与C1、C2、C4抗体水平无显著差异。
表4 免疫PRRS疫苗前后抗体变化
Table 4 Antibody changes before and after immunization with PRRS vaccine
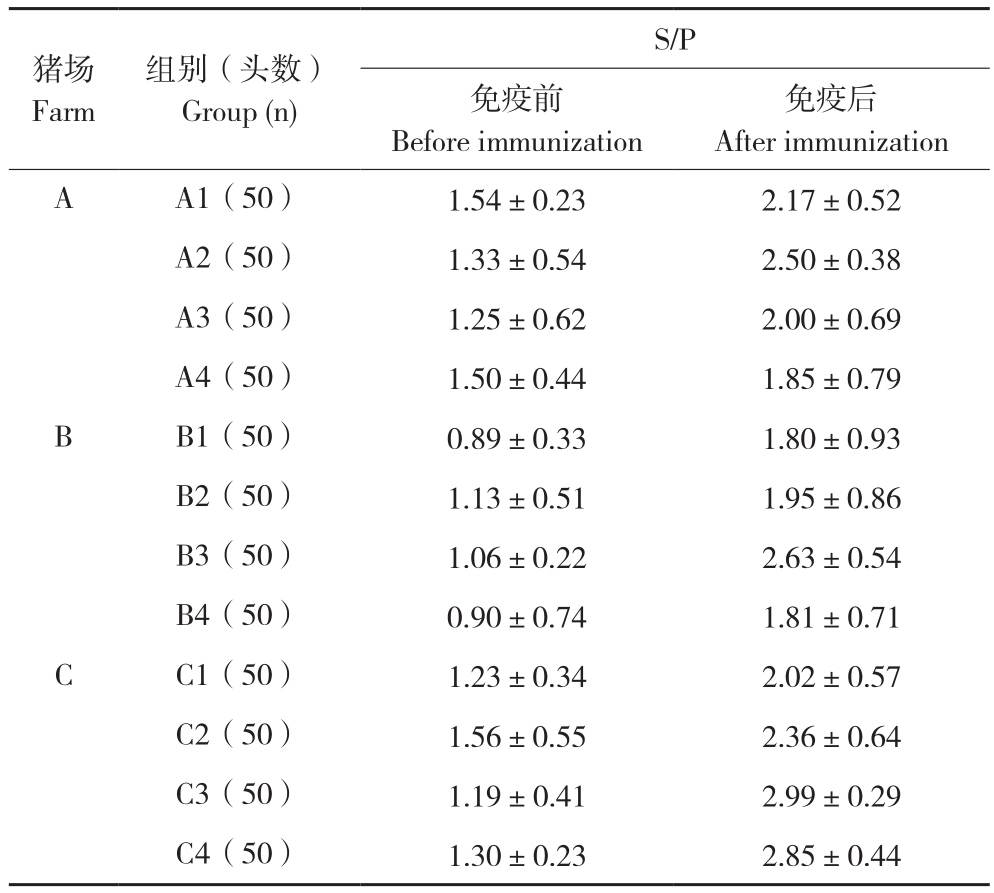
猪场Farm免疫后After immunization A A1(50) 1.54±0.23 2.17±0.52 A2(50) 1.33±0.54 2.50±0.38 A3(50) 1.25±0.62 2.00±0.69 A4(50) 1.50±0.44 1.85±0.79 B B1(50) 0.89±0.33 1.80±0.93 B2(50) 1.13±0.51 1.95±0.86 B3(50) 1.06±0.22 2.63±0.54 B4(50) 0.90±0.74 1.81±0.71 C C1(50) 1.23±0.34 2.02±0.57 C2(50) 1.56±0.55 2.36±0.64 C3(50) 1.19±0.41 2.99±0.29 C4(50) 1.30±0.23 2.85±0.44组别(头数)Group (n)S/P免疫前Before immunization
2.2 不同疫苗组合试验猪血清PRRSV核酸检测结果
猪血清中PRRSV核酸检测结果(表5)显示,A1~A4 组PRRSV核酸阳性率分别为16%(8/49)、12.5%(6/48)、18%(8/44)和33%(15/45);B1~B4组PRRSV核酸阳性率分别为10.6%(5/47)、0(0/48)、0(0/45)和13.6%(6/44);C1~C4组PRRSV核酸阳性率分别为6.3%(3/47)、8.5%(4/47)、10.8%(5/46)和18.1%(8/44)。
比较分析3个养殖场PRRSV核酸检测结果,第4组PRRSV核酸阳性率(A4=33%、B4=13.6%、C4=18.1%)与第1组PRRSV核酸阳性率(A1=16%、B1=10.6%、C1=6.3%)无显著差异,与第2组PRRSV核酸阳性率(A2=12.5、B2=0、C2=10.6%)有显著差异,与第3组PRRSV核酸阳性率(A3=18%、B3=0、C3=10.8%)也有显著差异。第1、2、3组PRRSV核酸阳性率两两分析无显著差异。
表5 不同疫苗组合试验猪血清PRRSV核酸检测结果
Table 5 Detection results of PRRSV nucleic acid in pig serum in different vaccination groups
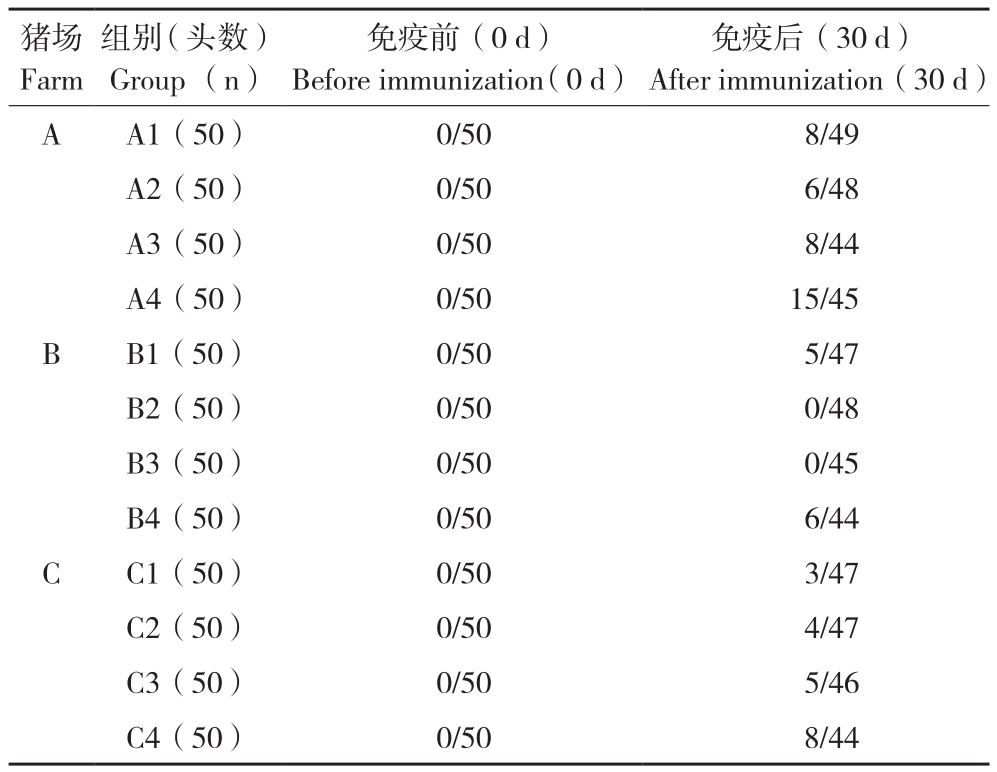
猪场Farm免疫后(30 d)After immunization(30 d)A A1(50) 0/50 8/49 A2(50) 0/50 6/48 A3(50) 0/50 8/44 A4(50) 0/50 15/45 B B1(50) 0/50 5/47 B2(50) 0/50 0/48 B3(50) 0/50 0/45 B4(50) 0/50 6/44 C C1(50) 0/50 3/47 C2(50) 0/50 4/47 C3(50) 0/50 5/46 C4(50) 0/50 8/44组别(头数)Group (n)免疫前(0 d)Before immunization(0 d)
2.3 不同疫苗组合试验猪生产参数比较
3个猪场4个不同免疫组合患呼吸道疾病仔猪头数分别为11、15、26、65头,发病率分别为7.3%、10%、17.3%和43.3%,仔猪死淘率分别为4.67%、4.67%、8.67%和11.33%。
3 讨论
目前我国市场上PRRS商品化疫苗在一定程度上对控制PRRS的传播和蔓延作出了贡献[20-23]。但由于PRRSV的生物性、抗原性和基因遗传多样性[24-27],使得不同毒株之间抗原的交叉保护有限,因此来源于某一分离毒株制备的疫苗不可能有效地保护猪群对抗具有抗原差异的PRRSV所有野毒株的感染[28-30]。因此,生产上常见免疫疫苗的猪群发生非典型和急性PRRS 病例。因此,开发安全有效的PRRS疫苗以及制定合理的免疫方案迫在眉睫[31-35]。
本研究为了探讨PRRS疫苗在PRRSV阳性场的免疫效果,选取3个PRRSV阳性场,采用不同疫苗毒株、不同疫苗组合(同时接种PRRS弱毒苗+灭活苗、先接种弱毒苗后接种灭活苗、先后接种两次弱毒苗、接种一次弱毒苗 4个组合)的免疫方案,通过对免疫后抗体水平变化、病毒血症和生产参数等方面评估疫苗免疫效果。
在当前PRRSV感染状态下,与单一疫苗免疫组合(14日龄免疫弱毒苗)相比,两针免疫组合(14日龄同时免疫PRRS弱毒苗和灭活苗、14日龄免疫PRRS弱毒苗和35日龄免疫灭活苗、14日龄免疫PRRS弱毒苗和35日龄免疫弱毒苗)能够产生更高的抗体反应。在病毒血症方面,与单一疫苗免疫组合(14日龄免疫弱毒苗)相比,两针免疫组合(14日龄免疫PRRS弱毒苗和35日龄免疫灭活苗、14日龄免疫PRRS弱毒苗和35日龄免疫弱毒苗)PRRSV核酸阳性率显著降低。在生产参数方面,与单一疫苗免疫组合(14日龄免疫弱毒苗)相比,两针免疫组合(14日龄同时免疫PRRS弱毒苗和灭活苗、14日龄免疫PRRS弱毒苗和35日龄免疫灭活苗、14日龄免疫PRRS弱毒苗和35日龄免疫弱毒苗)仔猪发病率、仔猪死淘率、呼吸道疾病的仔猪头数明显降低。
4 结论
本研究在3个PRRSV阳性猪场采用4种不同PRRS疫苗组合,通过对免疫后抗体水平变化规律、病毒血症和生产参数等方面评估疫苗免疫效果,结果表明, 14日龄同时免疫PRRS弱毒苗和灭活苗、14日龄免疫PRRS弱毒苗和35日龄免疫灭活苗两种免疫组合的免疫效果最好。这两种免疫组合可以为PRRSV阳性猪场的PRRS防控提供参考。
[1]WENSVOORT G,TERPSTRA C,Pol J M,TER LAAK E A,BLOEMRAADM,DE KLUYVER E P,KRAGTEN C,VAN BUITEN L,DEN BESTEN A,WAGENAAR F,Et Al.Mystery swine disease in The Netherlands: the isolation of Lelystad virus[J].The Veterinary Quarterly, 1991.13(3): 121-130.
[2]CHRISTIANSON W T,COLLINS J E,BENFIELD D A,HARRIS L,GORCYCA D E,CHLADEK D W,MORRISON R B,JOO H S.Experimental reproduction of swine infertility and respiratory syndrome in pregnant sows[J].American Journal of Veterinary Research,1992, 53(4): 485- 488.
[3]CHRISTIANSON W T,CHOI C S,COLLINS J E,MOLITOR T W,MORRISON R B,JOO H S.Pathogenesis of porcine reproductive and respiratory syndrome virus infection in mid-gestation sows and fetuses [J].Canadian Journal of Veterinary Research, 1993, 57(4): 262-268.
[4]ROSSOW K D,BAUTISTA E M,GOYAL S M,MOLITOR T W,MURTAUGH M P,MORRISON R B,BENFIELD D A,COLLINS J E.Experimental porcine reproductive and respiratory syndrome virus infection in one-, four-, and 10-week-old pigs[J].Journal of Veterinary Diagnostic Investigation, 1994, 6(1): 3-12.doi:10.1177/104063879400600102.
[5]LAGER K M,MENGELING W L.Pathogenesis of in utero infection in porcine fetuses with porcine reproductive and respiratory syndrome virus[J].Canadian journal of Veterinary Research, 1995, 59(3):187-192.
[6]MENGELING W L,VORWALD A C,LAGER K M,BROCKMEIER S L.Comparison among strains of porcine reproductive and respiratory syndrome virus for their ability to cause reproductive failure[J].American journal of veterinary research, 1996, 57(6): 834-839.
[7]蔡汝健,蒋智勇,刘燕玲,张乐宜,宋长绪.我国猪繁殖与呼吸综合征病毒的分子演化及与疫苗毒株的比较分析[J].广东农业科学,2013,40(8):115-120.doi: 10.16768/j.issn.1004-874X.2013.08.008.CAI R J, JIANG Z Y, LIU Y L, ZHAGN L Y, SONG C X.Molecular evolution comparison analysis between the field and vaccine strains of porcine reproductive and respiratory syndrome virus in China[J].Guangdong Agricultural Sciences, 2013,40(8):115-120.doi:10.16768/j.issn.1004-874X.2013.08.008.
[8]于林洋,董建国,张乐宜,刘燕玲,梁鹏帅,王 磊,宋长绪.猪繁殖与呼吸系统综合征病毒华南株GDgz 的分离鉴定及遗传进化分析[J].广东农业科学,2017,44(4):138-145.doi: 10.16768/j.issn.1004-874X.2017.04.021 YU L Y, DONG J G, ZHANG L Y, LIU Y L, LIANG P S, WANG L,SONG C X.Isolation, identification and genetic evolution of South China strain GDGZ of porcine reproductive and respiratory syndrome virus[J].Guangdong Agricultural Sciences, 2017,44(4):138-145.doi: 10.16768/j.issn.1004-874X.2017.04.021.
[9]张美玲,陆桂丽,薛晶,刘丽雅,简子健,夏俊.新疆猪繁殖与呼吸综合征病毒分离鉴定及Nsp2和ORF5基因遗传变异分析[J].广 东 农 业 科 学,2013, 40(7):106-110.doi:10.16768/j.issn.1004-874X.2014.17.001.ZHANG M L, LU G L, XUE L, LIU L Y, JIAN Z J, XIA J.Isolation and identification of Xinjiang porcine reproductive and respiratory syndrome virus and genetic variation analysis of Nsp2 and ORF5 genes[J].Guangdong Agricultural Sciences, 2013, 40(7):106-110.doi:10.16768/j.issn.1004-874X.2014.17.001.
[10]DEWEY C E,WILSON S,BUCK P,LEYENAAR J K.Effects of porcine reproductive and respiratory syndrome vaccination in breedingage animals[J].Preventive Veterinary Medicine, 2004, 62(4): 299-307.doi:10.1016/j.prevetmed.2003.11.007.
[11]SCORTTI M,PRIETO C,MARTINEZ-LOBO F J,SIMARRO I,CASTRO J M.Effects of two commercial European modified-live vaccines against porcine reproductive and respiratory syndrome viruses in pregnant gilts[J].Veterinary Journal, 2006, 172(3): 506-514.doi:10.1016/j.tvjl.2005.07.015.
[12]CANO J P,DEE S A,MURTAUGH M P,TRINCADO C A,PIJOAN C B.Effect of vaccination with a modified-live porcine reproductive and respiratory syndrome virus vaccine on dynamics of homologous viral infection in pigs[J].American Journal of Veterinary Research,2007, 68(5): 565-571.doi:10.2460/ajvr.68.5.565.
[13]ROSE N,RENSON P,ANDRAUD M,PABOEUF F,LE POTIER M F,BOURRY O.Porcine reproductive and respiratory syndrome virus(PRRSv)modified-live vaccine reduces virus transmission in experimental conditions[J].Vaccine, 2015, 33(21): 2493-2499.doi:10.1016/j.vaccine.2015.03.040.
[14]JEONG J,KIM S,PARK K H,KANG I,PARK S J,PARK C,CHAE C.Evaluation of the effect of a porcine reproductive and respiratory syndrome (PRRS) modified-live virus vaccine on sow reproductive performance in endemic PRRS farms[J].Veterinary Microbiology,2017, 208: 47-52.doi:10.1016/j.vetmic.2017.07.016.
[15]STADLER JULIA,ZOELS SUSANNE,EDDICKS MATTHIAS,KRAFT CHRISTIAN,RITZMANN MATHIAS,LADINIG ANDREA.Assessment of safety and reproductive performance after vaccination with a modified live-virus PRRS genotype 1 vaccine in pregnant sows at various stages of gestation[J].Vaccine, 2016, 34(33): 3862-3866.doi:10.1016/j.vaccine.2016.05.042.
[16]SCHELKOPF ADAM,NEREM JOEL,COWLES BOBBY,AMODIE DEB,SWALLA RICHARD,DEE SCOTT.Reproductive, productivity,and mortality outcomes in late-gestation gilts and their litters following simulation of inadvertent exposure to a modified-live vaccine strain of porcine reproductive and respiratory syndrome(PRRS)virus[J].Vaccine, 2014, 32(36): 4639-4643.doi:10.1016/j.vaccine.2014.06.073.
[17]NILUBOL D.The effect of a killed porcine reproductive and respiratory syndrome virus(PRRSV)vaccine treatment on virus shedding in previously PRRSV infected pigs[J].Veterinary Microbiology, 2004,102(1/2): 11-18.doi:10.1016/j.vetmic.2004.05.006.
[18]RENUKARADHYA GOURAPURA J,MENG XIANG-JIN,CALVERT JAY G,ROOF MICHAEL,LAGER KELLY M.Inactivated and subunit vaccines against porcine reproductive and respiratory syndrome: Current status and future direction[J].Vaccine, 2015, 33(27): 3065-3072.doi:10.1016/j.vaccine.2015.04.102.
[19]KARNIYCHUK U U,SAHA D,VANHEE M,GELDHOF M,CORNILLIE P,CAIJ A B,DE REGGE N,NAUWYNCK H J.Impact of a novel inactivated PRRS virus vaccine on virus replication and virus-induced pathology in fetal implantation sites and fetuses upon challenge[J].Theriogenology, 2012, 78(7): 1527-1537.doi:10.1016/j.theriogenology.2012.06.015.
[20]MADAPONG A,TEMEEYASEN G,SAENG-CHUTO K,TRIPIPAT T,NAVASAKULJINDA W,BOONSOONGNERN A,TANTITUVANONT A,NILUBOL D.Humoral immune responses and viral shedding following vaccination with modified live porcine reproductive and respiratory syndrome virus vaccines[J].Archives of Virologyl, 2017, 162(1):139-146.doi:10.1007/s00705-016-3084-4.
[21]CORREAS I,OSORIO F A, STEFFEN D,PATTNAIK A K,VU H L .Cross reactivity of immune responses to porcine reproductive and respiratory syndrome virus infection[J].Vaccine, 2017, 35(5): 782-788.doi:10.1016/j.vaccine.2016.12.040.
[22]MARTELLI PAOLO,CORDIOLI PAOLO,ALBORALI LORIS GIOVANNI,GOZIO STEFANO,DE ANGELIS ELENA,FERRARI LUCA,LOMBARDI GUERINO,BORGHETTI PAOLO.Protection and immune response in pigs intradermally vaccinated against porcine reproductive and respiratory syndrome(PRRS)and subsequently exposed to a heterologous European(Italian cluster)field strain[J].Vaccine, 2007, 25(17): 3400-3408.doi:10.1016/j.vaccine.2006.12.050.
[23]RAPPE J C,GARCIA-NICOLAS O,FLUCKIGER F,THUR B,HOFMANN M A,SUMMERFIELD A,RUGGLI N.Heterogeneous antigenic properties of the porcine reproductive and respiratory syndrome virus nucleocapsid[J].Veterinary Research, 2016,47(1):117.doi:10.1186/s13567-016-0399-9.
[24]DO H Q,TRINH, D T,NGUYEN T L,VU T T,THAN D D,VAN LO T,YEOM M,SONG D,CHOE S,AN D J,"LE VP".Molecular evolution of type 2 porcine reproductive and respiratory syndrome viruses circulating in Vietnam from 2007 to 2015[J].BMC Veterinary Research, 2016, 12(1): 256.doi:10.1186/s12917-016-0885-3.
[25]LEE J A,LEE N H,LEE J B,PARK S Y,SONG C S,CHOI I S,LEE S W.Genetic diversity of the Korean field strains of porcine reproductive and respiratory syndrome virus[J].Infection, Genetics and Evolution, 2016, 40: 288-294.doi:10.1016/j.meegid.2015.11.001.
[26]WU Q,LI Z,ZHANG G,NIU J,ZENG X,SUN B,MA J.Genetic diversity and phylogenetic analysis of porcine reproductive and respiratory syndrome virus in southern China from 2007 to 2014[J].Journal of Veterinary Science, 2017, 18(3):317-326.doi:10.4142/jvs.2017.18.3.317.
[27]BAI X,WANG Y,XU X,SUN Z,XIAO Y,JI G,LI Y,TAN F,LI X,TIAN K.Commercial vaccines provide limited protection to NADC30-like PRRSV infection[J].Vaccine, 2016, 34(46): 5540-5545.doi:10.1016/j.vaccine.2016.09.048.
[28]OUYANG K,HIREMATH J,BINJAWADAGI B,SHYU D L,DHAKAL S,ARCOS J,SCHLEAPPI R,HOLMAN L,ROOF M,TORRELLES J B,RENUKARADHYA G J.Comparative analysis of routes of immunization of a live porcine reproductive and respiratory syndrome virus(PRRSV)vaccine in a heterologous virus challenge study[J].Veterinary research, 2016, 47: 45.doi:10.1186/s13567-016-0331-3.
[29]CHOI K,PARK C,JEONG J,KANG I,PARK S J,CHAE C.Comparison of protection provided by type 1 and type 2 porcine reproductive and respiratory syndrome field viruses against homologous and heterologous challenge[J].The Veterinary Record, 2016, 191: 72-81.doi:10.1136/vr.103529.
[30]YU M,QIU Y,CHEN J,JIANG W.Enhanced humoral and cellular immune responses to PRRS virus GP5 glycoprotein by DNA primeadenovirus boost vaccination in mice[J].Virus Genes, 2016, 52(2):228-234.doi:10.1007/s11262-016-1293-2.
[31]JIANG W,JIANG P,LI Y,TANG J,WANG X,MA S.Recombinant adenovirus expressing GP5 and M fusion proteins of porcine reproductive and respiratory syndrome virus induce both humoral and cell-mediated immune responses in mice[J].Veterinary Immunology and Immunopathology, 2006, 113(1-2): 169-180.doi:10.1016/j.vetimm.2006.05.001.
[32]JIANG Y,XIAO S,FANG L,YU X,SONG Y,NIU C,CHEN H.DNA vaccines co-expressing GP5 and M proteins of porcine reproductive and respiratory syndrome virus(PRRSV)display enhanced immunogenicity[J].Vaccine, 2006, 24(15): 2869-2879.doi:10.1016/j.vaccine.2005.12.049.
[33]Huang Y W,Meng X J .Novel strategies and approaches to develop the next generation of vaccines against porcine reproductive and respiratory syndrome virus(PRRSV)[J].Virus Research, 2010, 154(1-2):141-149.doi:10.1016/j.virusres.2010.07.020.
[34]LU W,SUN B,MO J,ZENG X,ZHANG G,WANG L,ZHOU Q,ZHU L,LI Z,XIE Q,BI Y,MA J.Attenuation and immunogenicity of a live high pathogenic PRRSV vaccine candidate with a 32-amino acid deletion in the nsp2 protein[J].Journal of Immunology Research,2014: 810523.doi:10.1155/2014/810523.
[35]XIONG D,SONG L,ZHAI X,GENG S,PAN Z,JIAO X.A porcine reproductive and respiratory syndrome virus(PRRSV)vaccine candidate based on the fusion protein of PRRSV glycoprotein 5 and the Toll-like Receptor-5 agonist Salmonella Typhimurium FljB[J].BMC veterinary research, 2015(11): 121.doi:10.1186/s12917-015-0439-0.