【研究意义】水稻细菌性条斑病(bacterial leaf streak,BLS,简称细条病)是由黄单胞菌稻生致病变种(Xanthomonas oryzae pv. oryzicola,Xooc)侵染引起的细菌性病害[1]。细条病在我国是重要的检疫性病害之一[2-3],是继水稻稻瘟病、水稻纹枯病和水稻白叶枯病后第四大病害[4]。病原菌侵染植物后,植物体内将会激发相关的防御反应,而一系列保护酶活性的变化对植物自身抵御外来侵害发挥着重要作用[5]。因此,研究水稻感染细条病菌后酶活性的变化可为揭示水稻与病原菌互作机制提供依据,同时为水稻抗性育种提供参考。【前人研究进展】Shikanai等[6]发现烟草叶绿体中导入大肠杆菌的CAT基因,在光照强辐射即干旱胁迫下,转基因植物光合作用的耐光性明显高于对照;Polidoros等[7]发现烟草中转入玉米CAT 2基因,可以增强烟草对细菌的抑制能力,尽管过氧化氢酶(CAT)活性增加不显著;刘建宁等[8]选用抗旱性有差异的牧草幼苗作为材料,在0~72 h内测定其内部CAT活性,发现抗旱性弱材料体内的CAT活性大多时间低于抗旱性强材料;周明华等[9]通过对细条病侵染水稻抗感品种研究,发现苯丙氨酸解氨酶(PAL)活性与品种抗性呈负相关,原因尚待研究;李云锋等[10]发现过氧化物酶(POD)及PAL活性与植物的抗病性呈正相关,此指标可为植物抗性高低作鉴定,进而说明POD、PAL在植物抵抗病原菌过程中发挥重要作用;张晓葵等[11]通过研究细条病侵染抗感材料体内POD活性变化,发现感病品种POD活性下降,而抗性品种POD活性增强。王树彬等[12]研究表明,水稻叶片被细条病菌感染后感性品种叶片内CAT、SOD(超氧化物歧化酶)活性下降明显,POD活性略有上升,而抗病品种叶片内CAT、POD、SOD活性均显著上升。刘福等[13]研究发现,丛枝菌根真菌(Arbuscular mycorrhizal fungi,AMF)侵染易感黄萎病品种军棉1号后,丙二醛(MDA)含量受到抑制。目前,关于酚类物质与抗病性的关系研究结果尚无统一定论,如铃木直治[14]认为酚类物质酶活性变化与抗性不同的材料无直接影响,而周洁等[15]研究证明酚类物质酶活性与抗病性之间的关系成正相关。【本研究切入点】虽然在水稻与病原菌互作过程中植物酶活性的研究已取得一定进展,但对于各类植物酶在水稻抵抗细条病菌中所发挥的作用,还缺乏认识,关于Xooc侵染后抗、感近等基因系植株酶活性的变化仍少见报道。【拟解决的关键问题】通过测定Xooc侵染水稻细条病抗、感近等基因系后MDA含量以及CAT、PAL、POD、PPO(多酚氧化酶)和SOD在不同时间点叶片内的活性,明确Xooc侵染对水稻体内主要保护酶活性的影响,为研究和防治水稻细条病提供理论依据。
1 材料与方法
1.1 试验材料
以感病籼稻品种9311为轮回亲本,抗病野生稻材料DY19为供体亲本,通过杂交、回交和自交培育BC4F3代,获得水稻细条病抗病近等基因系LR19和感病近等基因系LS19。2019年9月种植LR19和LS19各100株于广西大学农学院科学研究试验基地。
供试菌株:水稻细条病菌(Xooc)采用广西水稻细条病优势生理小种 GX01,由广西大学亚热带农业生物资源保护与利用国家重点实验室何勇强教授提供。
主要仪器和试剂:TGL-2150台式微量高速冷冻离心机(四川蜀科仪器有限公司)、ELx405Select深孔板酶标仪、岛津UX620H电子天平、G9系列紫外可见分光光度计(上海让奇仪器科技有限公司)、上海精宏DK-S26双列六孔电热恒温水浴锅、TG16台式高速台式离心机(上海卢湘仪离心机仪器有限公司)、格瑞斯BD200-UL电解液移液器、石英比色皿(宜兴市晶科光学仪器有限公司)、研钵(北京豫维科技有限公司)、冰和蒸馏水。
1.2 试验方法
1.2.1 取样与接种 水稻种植采用盆栽法,盆大小为 60 cm×40 cm,每盆10行,每行10株,抗、感病近等基因系间行排列。做好水肥管理及病虫害防治工作,待材料长至分蘖期时接种取用。接种时选择生长较一致的叶片,每隔2~3 cm接种1对针刺点,取样时取3片这样接种的一整片叶片。于接种后0、24、48、72、96 h从接种Xooc(处理)和接种无菌水(对照)的植株上取样,样品做好标记保存于-80 ℃冰箱中。LS19-Xooc:LS19接种Xooc;LS19-CK:LS19接种无菌水(对照);LR19-Xooc:LR19接种Xooc;LR19-CK:LR19接种无菌水(对照)。
1.2.2 项目测定 样品采集后,采用北京索莱宝科技有限公司酶活测定试剂盒说明书进行粗酶液提取:0.1 g 水稻组织→1 mL提取液冰浴→8 000 r/min4 ℃离心10 min→取上清待测。按照试剂盒操作说明书步骤分别对CAT、MDA、PAL、POD、PPO、SOD在相应波长处的吸光值进行测定并计算,3次重复。
使用Excel 2019对数据进行整理,利用SPSS 22.0软件进行方差分析和t测验。
2 结果与分析
2.1 LR19和LS19受Xooc侵染后CAT活性变化
由图1可知,LR19和LS19在接菌0 h叶片内CAT活性分别为229.4、114.67 U/g,前者为后者的2倍,二者差异显著。接菌后0~96 h两个近等基因系CAT活性整体呈先升高后降低的趋势,且LR19始终高于LS19。接菌后0~48 h,LR19叶片内CAT活性迅速升高,达到峰值(336.37 U/g),较对照(179.34 U/g)增加87.56%;接菌后48~96 h,CAT活性随着时间延长而减少,接菌后96 h较对照(157.35 U/g)增加59.59%,差异显著;接菌后0~48 h,LS19叶片内CAT活性一直保持较高上升速率,在接菌48 h达到峰值(172.65 U/g),较对照(71.33 U/g)增加142.04%,差异显著;后期呈缓慢下降趋势,较对照(65.26 U/g)增加159.2%。综上所述,XooC侵染诱导抗、感病近等基因系后叶片内CAT活性增加,且前者的CAT活性始终高于后者,表明CAT活性与细条病抗性呈正相关。
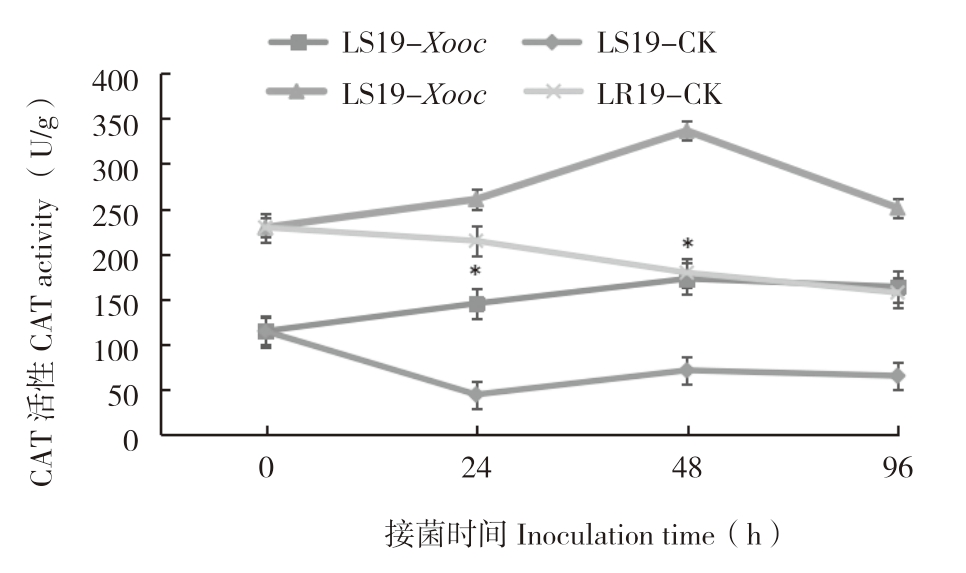
图1 LR19和LS19受Xooc侵染后叶片内CAT活性变化
Fig.1 Changes of CAT activity in leaves of LR19 and LS19 infected by Xooc
*表示接菌处理与对照相比差异显著
*asterisk represents significant difference between inoculated treatment and control treatment
2.2 LR19和LS19受Xooc侵染后MDA含量变化
由图2可知,LR19和LS19在接菌0 h叶片内MDA含量存在差异,分别为16.02、35.27 nmol/g,后者是前者的2.2倍。接菌后0~96 h,二者MDA含量整体呈下降趋势,且LR19大多低于LS19。LR19接菌后叶片内MDA含量减少,接菌后48 h MDA含量为4.90 nmol/g,较对照(20.39 nmol/g)减少75.97%,差异显著;接菌后48~96 h,MDA含量呈升高趋势,但总体仍低于对照,而对照叶片内MDA含量则趋于稳定;LS19接菌后叶片内MDA含量降低较快;接菌后48 h MDA含量为6.49 nmol/g,较对照(25.55 nmol/g)减少74.6%,差异显著;接菌后48~96 h呈升高趋势,接菌后96 h MDA含量达到10.6 nmol/g,较对照(22.52 nmol/g)减少52.93%,差异显著。综上所述,Xooc侵染导致抗、感病近等基因系后叶片内的MDA含量降低,且前者含量总体上低于后者,表明MDA含量积累与细条病抗性呈负相关。
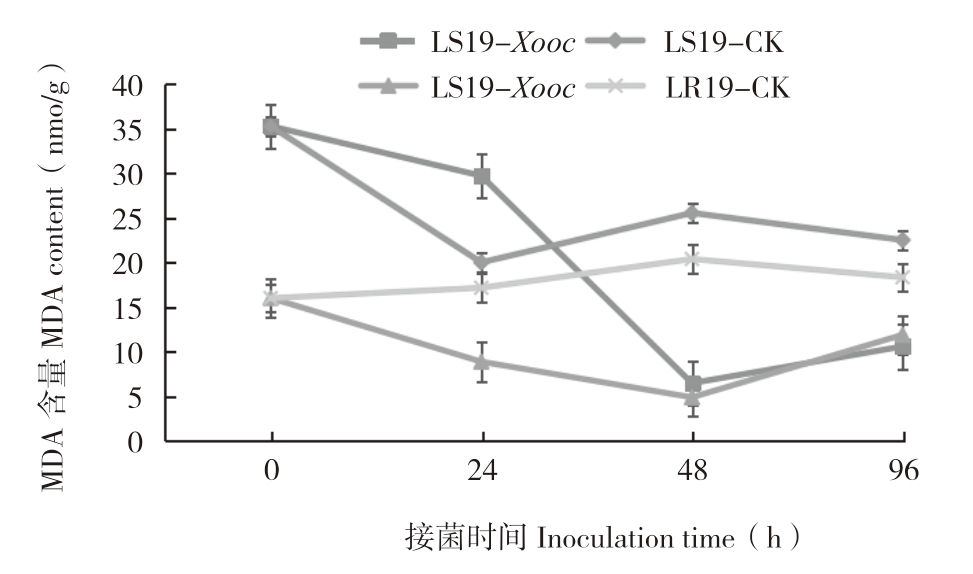
图2 LR19和LS19受Xooc侵染后叶片内MDA含量变化
Fig.2 Changes of MDA content in leaves of LR19 and LS19 infected by Xooc
“*”表示接菌处理与对照相比差异显著
The“*”asterisk represents significant difference between inoculated treatment and control treatment
2.3 LR19和LS19受Xooc侵染后PAL活性变化
由图3可知,接菌0 h,LR19和LS19叶片内PAL活性分别为30.5、27.2 U/g,差异不显著。接菌后0~96 h,抗、感近等基因系叶片内PAL活性均呈先升高后降低趋势,总体均表现升高。LR19接菌后0~48 h,PAL活性迅速升高并达到峰值(46.83 U/g),较对照(30.83 U/g)增加51.9%,差异显著,接菌后48 h PAL活性下降但总体仍高于0 h;LS19接菌0~48 h,PAL活性呈升高变化,接菌后48 h较对照叶片增加23.74%,后期开始缓慢下降,但仍高于对照,接菌后96 h较对照增加15.28%。综上所述,Xooc侵染诱导抗、感病近等基因系中PAL活性增强,且前者增强的幅度高于后者,表明PAL活性的增强有助于提高细条病抗性。
2.4 LR19和LS19受Xooc侵染后POD活性变化
由图4可知,接菌0 h,LR19和LS19叶片内POD活性分别为19 502、17 150 U/g,但差异不显著。接菌后0~96 h,抗、感近等基因系POD活性整体均表现为先升高后降低趋势,LR19始终高于LS19。接菌后0~48 h,LR19叶片内POD活性迅速上升,接菌后24 h,POD活性为34 741 U/g,较对照(24 108 U/g)差异显著;接菌后48 h POD活性达到峰值(38 752 U/g),较对照(15 876 U/g)增加144.09%,差异显著,接菌后48~96 h,POD活性表现为下降趋势,但仍高于对照;LS19接菌后0~48 h叶片内POD活性亦开始升高,接菌后24 h,POD活性为23 633 U/g,较对照(9 359 U/g)差异显著;接菌后48 h,POD活性升到峰值(34 496 U/g),较对照(5 716.7 U/g)增加503.43%,后期缓慢下降至31 262 U/g,较对照(9 800 U/g)增加219%,差异显著。综上所述,Xooc侵染诱导抗、感病近等基因系后叶片内的POD活性增加,且前者的POD活性始终高于后者,表明POD活性的增强有助于提高细条病抗性。
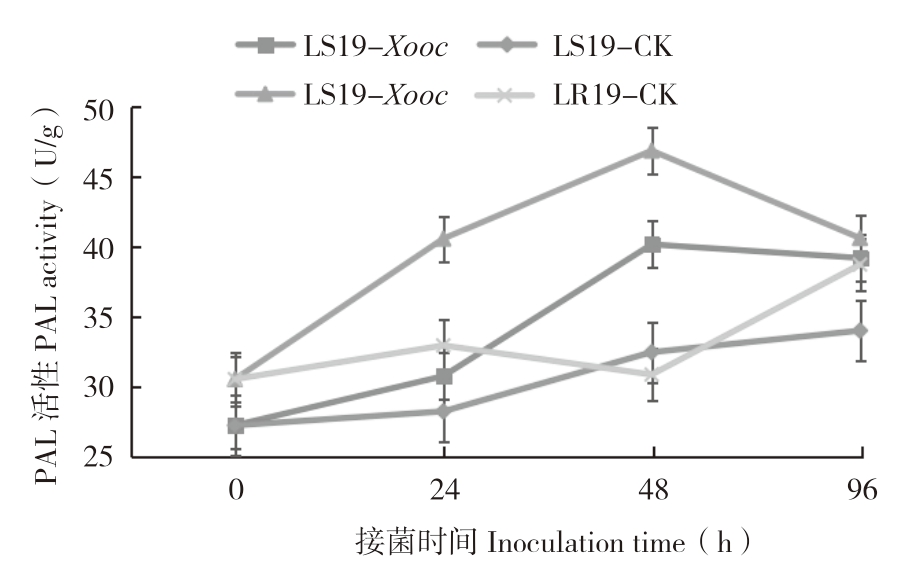
图3 LR19和LS19受Xooc侵染后叶片内PAL活性变化
Fig.3 Changes of PAL activity in leaves of LR19 and LS19 infected by Xooc
“*”表示接菌处理与对照相比差异显著
The“*”asterisk represents significant difference between inoculated treatment and control treatment
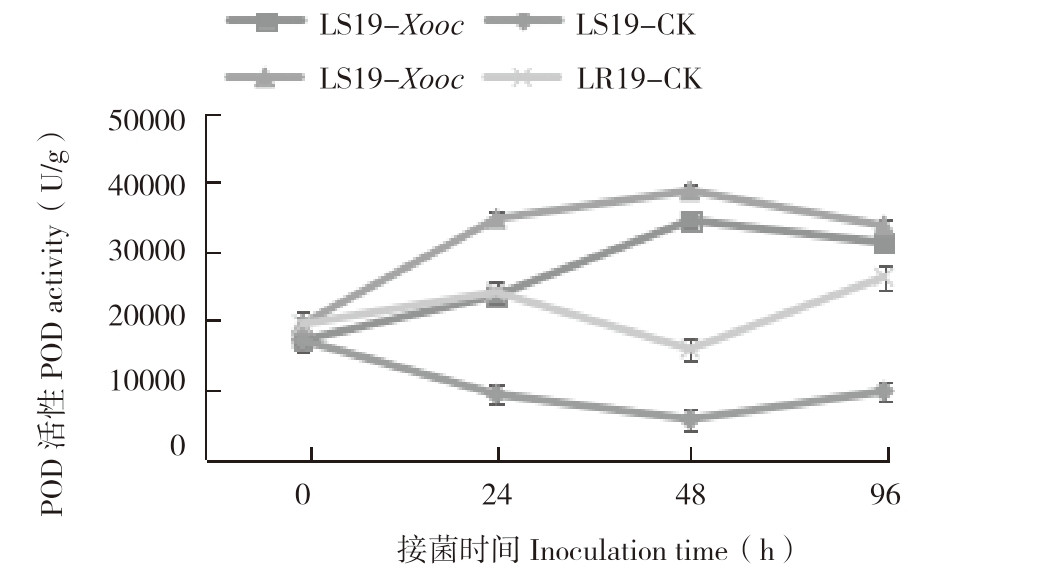
图4 LR19和LS19受Xooc侵染后叶片内POD活性变化
Fig.4 Changes of POD activity in leaves of LR19 and LS19 infected by Xooc
“*”表示接菌处理与对照相比差异显著
The“*”asterisk represents significant difference between inoculated treatment and control treatment
2.5 LR19和LS19受Xooc侵染后PPO活性变化
由图5可知,接菌0 h,LR19和LS19叶片内PPO活性分别为81.0、59.7 U/g,前者是后者的1.36倍。接菌后0~96 h,抗、感病近等基因系叶片内PPO活性呈先迅速上升后缓慢下降趋势,且抗病系始终高于感病系。LR19接菌后0~24 h PPO活性迅速升高并达到峰值(124.2 U/g),较对照(84.35 U/g)增加47.24%,差异显著;接菌24~96 h,PPO活性缓慢下降,仍高于对照;接菌后0~48 h LS19 PPO活性呈升高变化,48 h达到峰值(106.5 U/g),后期开始缓慢下降,但仍高于对照;接菌后96 h,PPO活性为98.5 U/g,较对照(58.7 U/g)增加67.8%,差异显著。综上所述,Xooc侵染诱导抗、感病近等基因系后叶片内的PPO活性增加,且前者的PPO活性增加较快,表明PPO活性的增强有助于提高细条病抗性。
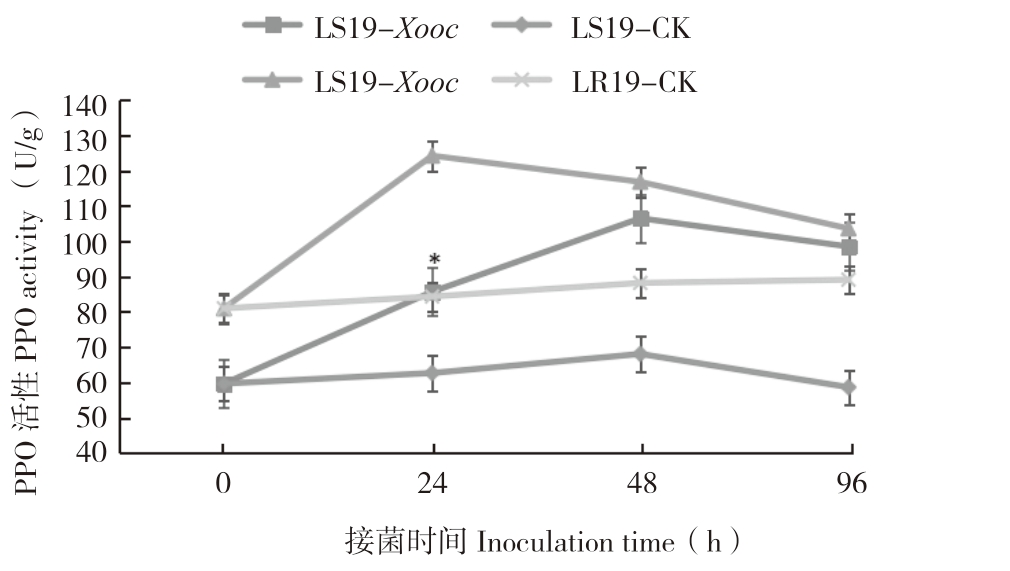
图5 LR19和LS19受Xooc侵染后叶片内PPO活性变化
Fig.5 Changes of PPO activity in leaves of LR19 and LS19 infected by Xooc
“*”表示接菌处理与对照相比差异显著
The“*”asterisk represents significant difference between inoculated treatment and control treatment
2.6 LR19和LS19受Xooc侵染后SOD活性变化
由图6可知,接菌0 h,LR19和LS19叶片内SOD活性差异不大,分别为976.53、840.20 U/g。接菌0~96 h,LR19叶片内SOD活性迅速升高,并整体保持在较高水平,而LS19叶片内SOD活性呈波动变化,但总体仍高于对照。接菌0~96 h,LR19的SOD活性始终高于LS19。接菌0~24 h,LR19叶片内SOD活性迅速升高至2 362.43 U/g,较对照(1 217.94 U/g)增加93.97%,接菌后48 h,SOD活性达到峰值(2 389.79 U/g),较对照(1 217.94 U/g)增加155.48%;接菌后48~96 h,SOD活性下降速率变快,仍高于对照;接菌后0~24 h,LS19叶片内SOD活性迅速升高;接菌后24 h,SOD活性为1 531.88 U/g,较对照(648.4 U/g)增加136.26%,差异显著;接菌后24 h,叶片内SOD活性呈下降趋势,仍高于对照;接菌后48~96 h,SOD活性升至1 824.51 U/g,较对照(571.75 U/g)增加219.11%,差异显著。综上所述,Xooc侵染诱导抗、感病近等基因系后叶片内的SOD活性增加,且前者SOD活性始终高于后者,表明SOD活性的增强有助于提高细条病抗性。
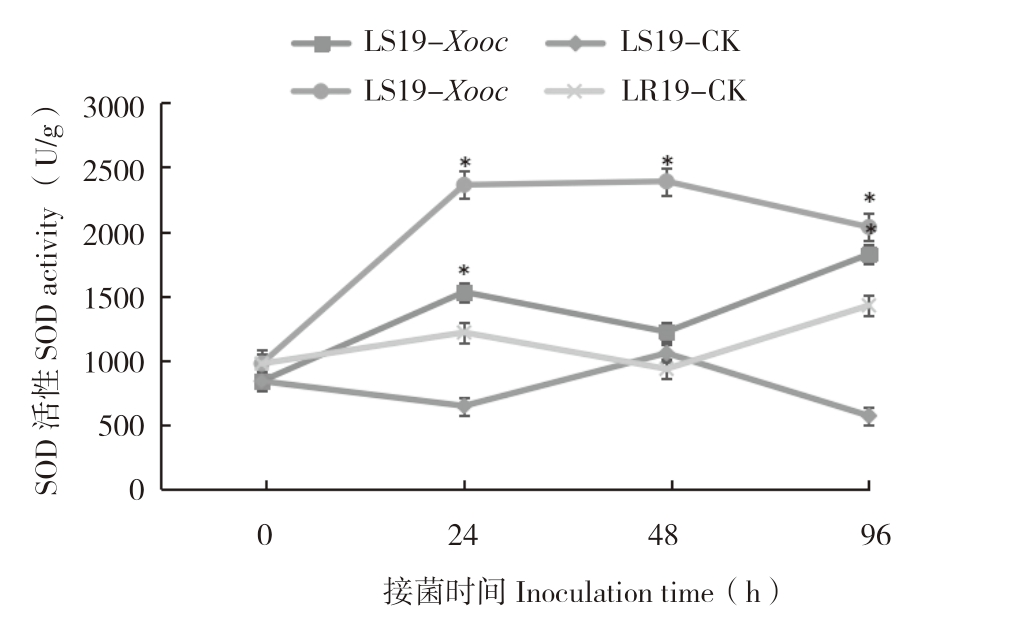
图6 LR19和LS19受Xooc侵染后叶片内SOD活性变化
Fig.6 Changes of SOD activity in leaves of LR19 and LS19 infected by Xooc
“*”表示接菌处理与对照相比差异显著
The“*”asterisk represents significant difference between inoculated treatment and control treatment
3 讨论
植物的防卫机制形成是在相关保护酶催化下一系列复杂生理生化代谢的结果[16]。尽管国内外许多学者深入研究了植物防御酶活性与抗病性的关系,可根据寄主与病原物互作体系不同,得出结论亦略有差异[17-18]。CAT是一种抗氧化酶,其活性在植物受到病原菌侵染时会发生变化,可用作衡量植物抗性反应的重要指标[19]。侯茜等[20]在西瓜幼苗根系防御酶活性变化与枯萎病抗性的关系的研究中,发现CAT活性与植物的抗病性呈正相关;孙正祥等[21]在内生菌 XG-1对西瓜枯萎病诱导抗性的研究中发现植物的抗病性与CAT息息相关。本研究对水稻抗、感近等基因系接种细条病菌后可知,抗、感材料在受到细条病菌侵染后,二者CAT活性均显著提高。该结果与魏滟洁等[22]在研究球毛壳菌与枯草芽孢杆菌组合侵染黄瓜后CAT活性变化结果一致。
MDA是膜脂过氧化反应过程中生成的的最终产物之一。江彤等[23]发现在烟草上接种黑胫病菌后抗性品种的MDA含量明显高于感病品种,且高于对照;孔祥华等[24]研究发现炭疽叶枯病菌诱导不同苹果种质中抗病品种富士MDA含量高于感病品种秦冠,且二者总体均表现升高趋势;而姚怀莲[25]在西瓜枯萎病抗性遗传及生理生化基础研究中发现MDA与抗性呈负相关。本研究结果表明,水稻抗、感近等基因系受到细条病菌侵染后,抗、感材料体内MDA含量均呈下降趋势,且感性材料的MDA含量高于抗性材料,表明MDA含量与水稻抗病性呈负相关。林英等[26]也得出相似结论。
PAL在植物抗病性物质生成途径即苯丙烷类代谢途径过程中既是限速酶也是关键酶[27],其活性增强对抗性物质木质素含量增加非常有利[28];其在植物抗病虫害反应中发挥重要作用,不仅参与植物抗病胁迫反应中相关物质的合成与积累,也是苯丙烷类代谢途径中最重要的酶,是衡量植物抗病反应过程中重要的生化指标[29-30]。本研究通过研究水稻抗、感近等基因系受细条病菌侵染后PAL活性变化发现,接种后抗、感材料PAL活性均显著升高,且抗性材料的升高幅度高于感性材料,表明PAL与水稻抗病性呈正相关。这与周东兴等[31]在研究番茄枯萎病生防细菌侵染番茄后PAL活性变化结果一致。
POD在植物体内普遍存在,是参与酚类物质、植保素及木质素合成的氧化还原酶,不仅参与植物代谢调节,且在植物抗病过程中发挥重要作用[32-33]。植物在受到病原菌侵染后,其体内活性氧代谢平衡遭受破坏,过多的活性氧不仅会破坏细胞结构使代谢系统紊乱,甚至会使植物细胞死亡[34],而POD是植物体内担负清除活性氧的关键酶[35]。本研究发现,细条病侵染水稻抗、感近等基因系后,抗性材料的POD活性增幅均显著高于感性材料,表明POD活性变化与水稻抗病性呈正相关关系。这与关峰等[36]研究结果相同。
植物的抗病性强弱一方面在于体内抗性基因是否表达,另一方面是基因表达后抗病反应的速度及抗病物质积累数量[37-38]。PPO氧化作用产生的醌类物质,如咖啡酸、绿原酸等是有效的杀菌物质,可使毒素失活或抑制病原菌毒素产生。同时,PPO是氧化生物体内酚类物质的主要酶类[39],邵正英等[40]以链霉菌JD211侵染水稻叶片,发现叶片受侵染后其体内PPO活性增强。本研究结果发现,水稻抗、感近等基因系受细条病菌侵染后PPO活性均呈升高趋势,抗性材料PPO活性显著高于感性材料。表明PPO活性的增强有助于提高抗病性。这与刘戈辉等[41]研究表达GhB301基因烟草和野生型烟草受到棉花枯萎病菌侵染后PPO活性变化的结果一致。
SOD属于植物抗氧化酶类,使活性氧的代谢水平维持平衡而防止过多活性氧产生对正常细胞造成不可逆的损伤[42],其在植物的抗逆境胁迫过程中起到重要保护作用[43]。任平等[44]通过研究猕猴桃植株接种猕猴桃溃疡病P-L菌株后SOD活性的变化发现,猕猴桃植株的抗病性与SOD活性的变化呈正相关关系。陈亮等[45]选用抗性材料PI296341-FR和感性材料Sugar Baby,分别接种西瓜枯萎病菌,发现SOD活性变化与西瓜抗病性密切相关,在抗西瓜枯萎生理活动中发挥着重要作用。本研究对抗、感近等基因系接种细条病菌后发现,抗感材料的SOD活性均升高,且抗性材料SOD活性始终高于感性材料,表明SOD活性与抗病性呈正相关,与前人研究一致。
4 结论
细条病菌侵染后,抗、感近等基因系CAT、PAL、POD、PPO、SOD活性均增强,且抗性近等基因系的活性始终高于感性近等基因系,表明这5种酶活性的增强有助于提高细条病抗性。细条病菌侵染会导致抗、感近等基因系中MDA含量降低,且抗性近等基因系的含量低于感性近等基因系,表明MDA含量的积累与细条病抗性呈负相关。因此这些酶的活性可以作为水稻细条病抗性鉴定的辅助评价指标。
[1] 方中达,任欣正,陈泰英,朱有釭,范怀忠,伍尚忠.水稻白叶枯病及条斑病和李氏禾条斑病病原细菌的比较研究[J].植物病理学报,1957,3(2):99-124.doi:10.13926/j.cnki.apps.1957.02.001.FANG Z D,REN X Z,CHEN T Y,ZHU Y H,FAN H Z,WU S Z.Comparative study on the bacterial bacteria of rice bacterial leaf blight and stripe leaf disease[J].Journal of Plant Pathology,1957,3(2):99-124.doi:10.13926/j.cnki.apps.1957.02.001.
[2] NINO L D,RONALD P C,BOGDANOVE A.Xanthomonas oryzae pathovars:model pathogens of a model crop[J].Molecular Plant Pathology,2010,7(5):303-324.doi:10.1111/j.1364-3703.2006.00344.
[3] 许志刚,钱菊梅.水稻细菌性条斑病适生性与控制研究进展[J].植物检疫,1995,9(4):239-244.doi:10.19662/j.cnki.issn1005-2755.XU Z G,QIAN J M.Research progress on the adaptability and control of rice bacterial stripe[J].Phytosanitary,1995,9(4):239-244.doi:10.19662/j.cnki.issn1005-2755.
[4] 陈玉奇,余明志,乐承伟,黄新燊.水稻细条病发生程度与损失率的关系[J].植物保护,1990,16(4):52.CHEN Y Q,YU M Z,LE C W,HUANG X S.Relationship between incidence of rice thin stripe disease and loss rate[J].Plant Protection,1990,16(4):52.
[5] 陈鹏,李振歧.BTH诱导小麦对白粉病的抗性与几丁质酶和β-1,3-葡聚糖酶活性诱导的关系[J].西北农林科技大学学报(自然科学版),2007(7):137-140.CHEN P,LI Z Q.Relationship between BTH-induced resistance to wheat powdery mildew and induction of chitinase and β-1,3-glucanase activity[J].Journal of Northwest A&F University(Natural Science Edition),2007(7):137-140.
[6] SHIKANAI T,YAMADA Y.Properties of the circular plasmid-like DNA,B4,from mitochondria of cytoplasmic male-sterile rice[J].Current Genetics,1988,13(5).:441.
[7] POLIDOROS A N,MYLONA P V,SCANDALIOS J G.Transgenic tobacco plants expressing the maize Cat2 gene have altered catalase levels that affect plant-pathogen interactions and resistance to oxidative stress[J].Transgenic Research,2001,10(6):555-569.
[8] 刘建宁,赵美清,王运琦,郭璞,李宝.水分胁迫对2种牧草幼苗脯氨酸及过氧化氢酶活性的影响[J].山西农业科学,2009,37(6):27-29.LIU J N,ZHAO M Q,WANG Y Q,GUO P,LI B.Effects of water stress on proline and catalase activities of two forage seedlings[J].Shanxi Agricultural Sciences,2009,37(6):27-29.
[9] 周明华,许志刚,沈秀萍.水稻品种与水稻细菌性条斑病菌的互作机制[J].植物保护学报,2001(2):103-107.ZHOU M H,XU Z G,SHEN X P.Interaction mechanism between rice varieties and bacterial bacterial leaf spot disease[J].Journal of Plant Protection,2001(2):103-107.
[10] 李云锋,王振中,贾显禄.稻瘟菌激发子CSBⅠ诱导水稻防御性相关酶的活性变化[J].作物学报,2004(6):613-617.LI Y F,WANG Z Z,JIA X L.Magnaporthe grisea elicitor CSBⅠ induces changes in the activities of defense-related enzymes in rice[J].Acta Agriculturae Sinica,2004(6):613-617.
[11] 张晓葵,王保仁,邓江明.水稻品种对稻细菌性条斑病菌的抗性与过氧化物酶、多酚氧化酶及酯酶同工酶的关系[J].湖南农业科学,1994(4):39-40.ZHANG X K,WANG B R,DENG J M.The relationship between the resistance of rice varieties to the bacterial leaf spot pathogen and the peroxidase, polyphenol oxidase and esterase isozymes[J].Hunan Agricultural Sciences,1994(4):39-40.
[12] 王树彬,叶明志,柯玉琴,伍迪明.水稻幼苗感染细菌性条斑病后细胞内几种保护性酶活性的变化[J].福建省农科院学报,1996(2):46-52.WANG S B,YE M Z,KE Y Q,WU D M.Changes of several protective enzyme activities in cells of rice seedlings infected with bacterial stripe[J].Journal of Fujian Academy of Agricultural Sciences,1996(2):46-52.
[13] 刘福,尉敬涛,王宇宏,余柯佳,王翔.丛枝菌根真菌(AMF)对棉花抗病防御酶系活性影响的研究[J].山西科技,2018,33(1):29-33.LIU F,YU J T,WANG Y H,YU K J,WANG X.Study on the effect of arbuscular mycorrhizal fungi (AMF) on the activity of cotton disease-resistant defense enzymes[J].Shanxi Science and Technology,2018,33(1):29-33.
[14] 铃木直治.近代植物病理化学[M].上海:上海科学技术出版社,1985.LING M Z Z.Modern plant pathology chemistry[M].Shanghai:Shanghai Science and Technology Press,1985.
[15] 周洁,陈伟,叶明志,夏怡厚.水稻感染细菌性条斑病后叶片中酚类物质的变化[J].福建农业大学学报,1997(2):250-255.ZHOU J,CHEN W,YE M Z,XIA Y H.Changes of phenolic substances in rice leaves after infection with bacterial stripe[J].Journal of Fujian Agricultural University,1997(2):250-255.
[16] 胡婷婷.栽培基质对几种蔬菜有机生态型无土栽培的影响[D].延吉:延边大学,2015.HU T T.Effects of cultivation medium on organic ecological soilless cultivation of several vegetables[D].Yanji:Yanbian University, 2015.
[17] 刘会宁,李从玉.6个生理生化指标与葡萄抗白粉病的关系[J].中国南方果树,2015,44(5):79-82.LIU H N,LI C Y.The relationship between 6 physiological and biochemical indexes and grape powdery mildew resistance[J].South China Fruit Trees,2015,44(5):79-82.
[18] 王雅雅.甜瓜土传叶枯病拮抗菌的选育及其生防潜力的评价[D].西安:西北大学,2015.WANG Y Y.Breeding of antagonistic antibacterial of melon soil-borne leaf blight and evaluation of its biocontrol potential[D].Xian:Northwest University,2015.
[19] 刘长命,杨瑞平,莫言玲,王永琦,郑俊鶱,张显.外源Spd预处理对甜瓜白粉病抗性及其内源多胺的诱导分析[J].西北植物学报,2016,36(1):85-92.doi:10.7606/j.issn.1000-4025.2016.01.0085.LIU C M,YANG R P,MO Y L,WANG Y Q,ZHENG J Q,ZHANG X.Exogenous spd pretreatment for melon powdery mildew resistance and induced analysis of endogenous polyamines[J].Journal of Northwestern Plants,2016,36(1):85-92.doi:10.7606/j.issn.1000-4025.2016.01.0085.
[20] 侯茜,羊杏平,张曼,徐锦华,刘广.西瓜幼苗根系防御酶活性变化与枯萎病抗性的关系[J].江苏农业科学,2015,43(12):147-149.doi:10.15889/j.issn.1002-1302.2015.12.044.HOU X,YANG X P,ZAHNG M,XU J H,LIU G.The relationship between the change of defense enzyme activity in watermelon seedling root system and resistance to Fusarium wilt[J].Jiangsu Agricultural Sciences,2015,43(12):147-149.doi:10.15889/j.issn.1002-1302.2015.12.044.
[21] 孙正祥,王丰,周燚.生防菌XG1对西瓜根际微生物群落及酶活的影响[J].河南农业科学,2013,42(4):107-110.doi:10.15933/j.cnki.1004-3268.2013.04.019.SUN Z X,WANG F,ZHOU Y.Effect of biocontrol bacteria XG1 on microbial community and enzyme activity of watermelon rhizosphere[J].Henan Agricultural Sciences,2013,42(4):107-110.doi:10.15933/j.cnki.1004-3268.2013.04.019.
[22] 魏滟洁,田叶韩,王炎峰,高克祥.球毛壳菌与枯草芽孢杆菌组合对抗黄瓜枯萎病防御酶活性的影响[J].山东农业科学,2019,51(7):72-79.doi:10.14083/j.issn.1001-4942.2019.07.016.WEI Y J,TIAN Y H,WANG Y F,GAO K X.The effect of the combination of chaetomium globosum and bacillus subtilis on the defensive enzyme activity against cucumber Fusarium wilt[J].Shandong Agricultural Sciences,2019,51(7):72-79.doi:10.14083/j.issn.1001-4942.2019.07.016.
[23] 江彤,杨建卿,高明,孔俊.不同抗病性烟草罹黑胫病后几种酶的活性及丙二醛含量的变化[J].安徽农业大学学报,2006(2):218-221.doi:10.13610/j.cnki.1672-352x.2006.02.018.JIANG T,YANG J Q,GAO M,KONG J.Changes of the activities of several enzymes and the content of malondialdehyde after different disease resistant tobaccos suffered from black shin disease[J].Journal of Anhui Agricultural University,2006(2):218-221.doi:10.13610/j.cnki.1672-352x.2006.02.018.
[24] 孔祥华,侯董亮,张伟,田义轲,刘源霞,王彩虹.炭疽叶枯病菌诱导的不同苹果种质中防御酶活性及丙二醛含量比较[J].青岛农业大学学报(自然科学版),2017,34(1):5-8.KONG X H,HOU D L,ZHANG W,TIAN Y K,LIU Y X,WANG C H.Comparison of defensive enzyme activity and malondialdehyde content in different apple germplasms induced by Anthracnose leaf blight[J].Journal of Qingdao Agricultural University(Natural Science Edition),2017,34(1):5-8.
[25] 姚怀莲.西瓜枯萎病抗性遗传及生理生化基础研究[D].扬州:扬州大学,2007.YAO H L.Watermelon Fusarium wilt resistance genetics and basic physiological and biochemical research[D].Yangzhou:Yangzhou University,2007.
[26] 林英,张海东,谢瑾卉,刘欣宇,臧超群,于舒怡,黄玉茜,梁春浩.花生褐斑病菌侵染对不同抗性花生活氧代谢及防御酶的影响[J].湖北农业科学,2018,57(16):51-56.doi:10.14088/j.cnki.issn0439-8114.2018.16.012.LIN Y,ZHANG H D,XIE J H,LIU X Y,ZANG C Q,YU S Y,HUANG Y X,LIANG C H.Effect of peanut brown spot pathogen infection on oxygen metabolism and defensive enzymes of different resistant flowers[J].Hubei Agricultural Sciences,2018,57(16):51-56.doi:10.14088/j.cnki.issn0439-8114.2018.16.012.
[27] 陈亮,陈年来.西瓜叶片防御酶活性与枯萎病抗性的关系[J].河南农业科学,2019,48(1):77-83,114.doi:10.15933/j.cnki.1004-3268.2019.01.012.CHEN L,CHEN N L.Relationship between watermelon leaf defense enzyme activity and resistance to wilt disease[J].Henan Agricultural Sciences,2019,48(1):77-83,114.doi:10.15933/j.cnki.1004-3268.2019.01.012.
[28] 许勇,王永健,葛秀春,宋凤鸣,郑重.枯萎病菌诱导的结构抗性和相关酶活性的变化与西瓜枯萎病抗性的关系[J].果树科学,2000(2):123-127.doi:10.13925/j.cnki.gsxb.2000.02.010.XU Y,WANG Y J,GE X C,SONG F M,ZHENG Z.Relationship between structural resistance induced by Fusarium oxysporum f.sp.and related enzyme activity and watermelon wilt resistance[J].Fruit Tree Science,2000(2):123-127.doi:10.13925/j.cnki.gsxb.2000.02.010.
[29] 史芳芳,孟伟芳,张现丽,刘艺平,孔德政.腐烂病菌对不同抗性荷花品种防御酶活性的影响[J].河南科学,2016,34(12):1997-2001.SHI F F,MENG W F,ZHANG X L,LIU Y P,KOMG D Z.Effects of rot pathogens on defense enzyme activities of different resistant lotus varieties[J].Henan Science,2016,34(12):1997-2001.
[30] 翟彩霞,马春红,秦君,王立安,陈霞,李广敏.植物诱导抗病性的常规鉴定——相关酶活性变化与诱导抗病性的关系[J].中国农学通报,2004(5):222-224.ZHAI C X,MA C H,QIN J,WANG L A,CHEN X,LI G M.Routine identification of plant induced disease resistance—Relationship between changes in related enzyme activities and induced disease resistance[J].Chinese Agricultural Science Bulletin,2004(5):222-224.
[31] 周东兴,王恩泽,刘多,金聪敏,李欣,姜姗,白皓天.番茄枯萎病生防细菌的筛选及对植株防御酶活性的影响[J].生态学杂志,2020,39(5):1753-1760.doi:10.13292/j.1000-4890.202005.004.ZHOU D X,WANG E Z,LIU D,JIN C M,LI X,JIANG S,BAI H T.Screening of biocontrol bacteria against tomato Fusarium wilt and its effect on plant defense enzyme activities[J].Journal of Ecology,2020,39(05):1753-1760.doi:10.13292/j.1000-4890.202005.004.
[32] 史芳芳,孟伟芳,张现丽,刘艺平,孔德政.腐烂病菌对不同抗性荷花品种防御酶活性的影响[J].河南科学,2016,34(12):1997-2001.SHI F F,MENG W F,ZHANG X L,LIU Y P,KOMG D Z.Effects of rot pathogens on defense enzyme activities of different resistant lotus varieties[J].Henan Science,2016,34(12):1997-2001.
[33] 董汉松,王金生,方中达.细菌凝集素与菌体脂多糖在大白菜与软腐欧氏杆菌接触识别中的作用[J].植物病理学报,1995(1):51-56.DONG H S,WANG J S,FANG Z D.The role of bacterial lectins and bacterial lipopolysaccharides in contact recognition between Chinese cabbage and Erwinia soft-rot[J].Journal of Plant Pathology,1995(1):51-56.
[34] 陈亮,陈年来.西瓜叶片防御酶活性与枯萎病抗性的关系[J].河南农业科学,2019,48(1):77-83,114.doi:10.15933/j.cnki.1004-3268.2019.01.012.CHEN L,CHEN N L.Relationship between watermelon leaf defense enzyme activity and resistance to wilt disease[J].Henan Agricultural Sciences,2019,48(1):77-83,114.doi:10.15933/j.cnki.1004-3268.2019.01.012.
[35] 邹芳斌,司龙亭,李新,王莉莉.黄瓜枯萎病抗性与防御系统几种酶活性关系的研究[J].华北农学报,2008(3):181-184.ZOU F B,SI L T,LI X,WANG L L.Study on the relationship between cucumber wilt resistance and several enzyme activities in defense system[J].North China Agricultural Journal,2008(3):181-184.
[36] 关峰,张景云,石博,万新建.苦瓜枯萎病抗性鉴定及枯萎病菌胁迫下生理响应差异分析[J].植物生理学报,2019,55(10):1481-1488.doi:10.13592/j.cnki.ppj.2019.0234.GUAN F,ZHANG J Y,SHI B,WAN X J.Identification of resistance to Fusarium wilt of balsam pear and analysis of the difference of physiological response under Fusarium wilt stress[J].Acta Phytophysiology,2019,55(10):1481-1488.doi:10.13592/j.cnki.ppj.2019.0234.
[37] 陈年来,乃小英,张玉鑫,乔昌萍,张建农,李喜娥.植物源诱导剂对甜瓜叶片防卫酶活性的影响[J].西北植物学报,2010,30(10):2016-2021.CHEN N L,NAI X Y,ZHANG Y X,QIAO C P,ZHANG J N,LI XI E.Effects of plant-derived inducers on defensive enzyme activities of melon leaves[J].Journal of Northwestern Plants,2010,30(10):2016-2021.
[38] 陈亮,陈年来.西瓜叶片防御酶活性与枯萎病抗性的关系[J].河南农业科学,2019,48(1):77-83,114.doi:10.15933/j.cnki.1004-3268.2019.01.012.CHEN L,CHEN N L.Relationship between watermelon leaf defense enzyme activity and resistance to wilt disease[J].Henan Agricultural Sciences,2019,48(01):77-83,114.doi:10.15933/j.cnki.1004-3268.2019.01.012.
[39] 房保海,张广民,迟长凤,刘萍.烟草低头黑病菌毒素对烟草丙二醛含量和某些防御酶的动态影响[J].植物病理学报,2004(1):27-31.doi:10.13926/j.cnki.apps.2004.01.005.FANG B H,ZHANG G M,CHI C F,LIU P.The dynamic effect of the black toad black toxin on the content of tobacco malondialdehyde and certain defensive enzymes[J].Journal of Plant Pathology,2004(1):27-31.doi:10.13926/j.cnki.apps.2004.01.005.
[40] 邵正英,聂丽,徐志荣,李张,傅雁辉,魏赛金.链霉菌JD211对水稻酚类物质及相关酶活的影响[J].江西农业大学学报,2017,39(5):983-988.doi:10.13836/j.jjau.2017126.SHAO Z Y,NIE L,XU Z R,LI Z,FU Y H,WEI S J.Effect of streptomyces JD211 on rice phenols and related enzyme activities[J].Journal of Jiangxi Agricultural University,2017,39(5):983-988.doi:10.13836/j.jjau.2017126.
[41] 刘戈辉,朱金成,郭文婷,张鹏飞,张薇.转GhB301基因烟草的防御酶活性及抗病相关基因表达分析[J].西北植物学报,2019,39(11):2011-2018.doi:10.7606/jissn.1000-4025.2019.11.2011.LIU G H,ZHU J C,GUO W T,ZHANG P F,ZHANG W.Analysis of defensive enzyme activity and disease resistance-related gene expression of GhB301 transgenic tobacco[J].Acta Botanica Northwest,2019,39(11):2011-2018.doi:10.7606/jissn.1000-4025.2019.11.2011.
[42] 宋培玲,张键,郝丽芬,皇甫海燕,袁喜丽,包玉英,李子钦.不同抗性油菜品种接种黑胫病菌防御酶活性变化研究[J].华北农学报,2015,30(2):110-115.SONG P L,ZHANG J,HAO L F,HUANG F H Y,YUAN X L,BAO Y Y,LI Z Q.Study on the change of defense enzyme activity of black-leg disease inoculated with different resistant rape varieties[J].Journal of North China Agriculture,2015,30(2):110-115.
[43] 张蕊.水杨酸对低温胁迫下水稻幼苗过氧化氢与抗氧化酶系的影响研究// 2005年全国植物逆境生理与分子生物学研讨会论文摘要汇编[C].乌鲁木齐:2005.ZHANG R.Effects of salicylic Acid on hydrogen peroxide and antioxidant enzymes in rice seedlings under low temperature stress//A compilation of abstracts from the 2005 national symposium on plant stress physiology and molecular biology[C].Urumqi:2005.
[44] 任平,阮祥稳,赵文娟,秦涛.猕猴桃溃疡病P-L菌株诱导植株系统的抗性[J].江苏农业科学,2017,45(21):109-111.doi:10.15889/j.issn.1002-1302.2017.21.029.R EN P,RUAN X W,ZHAO W J,QIN T.Resistance of kiwi fruit canker P-L strain induced plant system[J].Jiangsu Agricultural Sciences,2017,45(21):109-111.doi:10.15889/j.issn.1002-1302.2017.21.029.
[45] 陈亮,陈年来.西瓜叶片防御酶活性与枯萎病抗性的关系[J].河南农业科学,2019,48(1):77-83,114.doi:10.15933/j.cnki.1004-3268.2019.01.012.CHEN L,CHEN N L.Relationship between watermelon leaf defense enzyme activity and resistance to wilt disease[J].Henan Agricultural Sciences,2019,48(1):77-83,114.doi:10.15933/j.cnki.1004-3268.2019.01.012.