文章信息
基金项目
- 广东省自然科学基金项目(2020A1515011391);广东省农业科学院科技人才引进项目(粤农科人〔2019〕25号);广东省农业科学院作物研究所所长基金/广东省农作物遗传改良重点实验室开放基金项目(202002)
作者简介
- 邵秀红(1983—),女,博士,助理研究员,研究方向为烟草资源功能成分与综合利用,E-mail:xhshao2007@126.com.
通讯作者
- 黄振瑞(1980—),男,博士,研究员,研究方向为烟草综合利用,E-mail:fjsi@163.com.
文章历史
- 收稿日期:2023-06-06
2. 广东烟草韶关市有限公司乳源瑶族自治县分公司,广东 韶关 512700
2. Ruyuan Yao Autonomous County Branch, Guangdong Tobacco Shaoguan Co., Ltd., Shaoguan 512700, China
绿原酸(Chlorogenic acid,CGA)是植物细胞通过莽草酸途径合成的一种物质,是植物体内重要的次生代谢产物,它与咖啡酸、没食子酸、原儿茶酸、阿魏酸、芥子酸等都是酚酸类化合物,而酚酸类与槲皮素、芦丁、木犀草素、花青素、表儿茶素等均属于多酚类化合物,这些物质大多具有较高的生物活性。绿原酸在金银花、杜仲、绿咖啡豆、茶叶等植物中含量很高[1],其中,金银花的标志性成分之一即是绿原酸。绿原酸不仅在植物生长发育、抗病虫害、抗寒等方面发挥重要作用,还具有多种药理功能,包括抗多种病原真菌,抗肺炎链球菌、金葡球菌和痢疾志贺氏菌等细菌,抑制肝癌、肺癌和胶质瘤等肿瘤,抗甲型流感、乙型肝炎、艾滋病等病毒,抗氧化和抗炎等,具有较强的肝功能和神经系统保护作用,对肥胖、血脂异常、糖尿病、高血压等代谢性疾病也有显著疗效[1-2]。因此,绿原酸在食品行业已有较多应用,它可作为水产品和肉制品的抗菌和抗氧化剂,延长其保质期;可作为水果保鲜剂,可防止饮料腐败变质;还可作为天然的食品添加剂,用于制作生产具有保健功效的食品。在医药行业,绿原酸是多种具有抗菌、消炎、解毒作用中成药的主要成分,也作为抗肿瘤药物用于晚期复发脑胶质母细胞瘤的II期临床研究[1]。因其突出的抗氧化作用,绿原酸可降低紫外线对人体皮肤的伤害,在美白、防晒、润肤等日用化工领域被广泛应用。随着绿原酸生物活性研究的不断深入,其在食品、医药和化工等领域的应用将更加广泛[3]。目前,绿原酸的主要来源是从天然植物中提取,如金银花、杜仲、绿咖啡豆等,然而随着全球绿原酸行业的发展,需要开发利用更多的植物资源,如农作物副产物,从废弃物综合利用的角度开发绿原酸提取新资源,将产生巨大的生态效益和经济效益。茄科植物如番茄、茄子、辣椒、马铃薯和烟草等,均是重要的经济作物,它们的果实或营养器官被人们食用或利用后,可为人们提供多种抗氧化剂,包括绿原酸等多酚类物质。前人已对绿原酸进行了较多的研究,对绿原酸的提取纯化、检测方法、生物活性、药理作用等方面也有较多的综述,而对于绿原酸在以上5种茄科作物中的含量和调控机制等方面尚缺少相关的综述报道。本文概述了近年来关于茄科作物中绿原酸的含量情况、生物合成途径及调控机制,以及绿原酸含量影响因素等方面的研究进展,并对其今后的研究重点进行展望,旨在为茄科作物绿原酸的合理开发利用、高绿原酸种质资源创制以及新品种培育等提供理论依据。
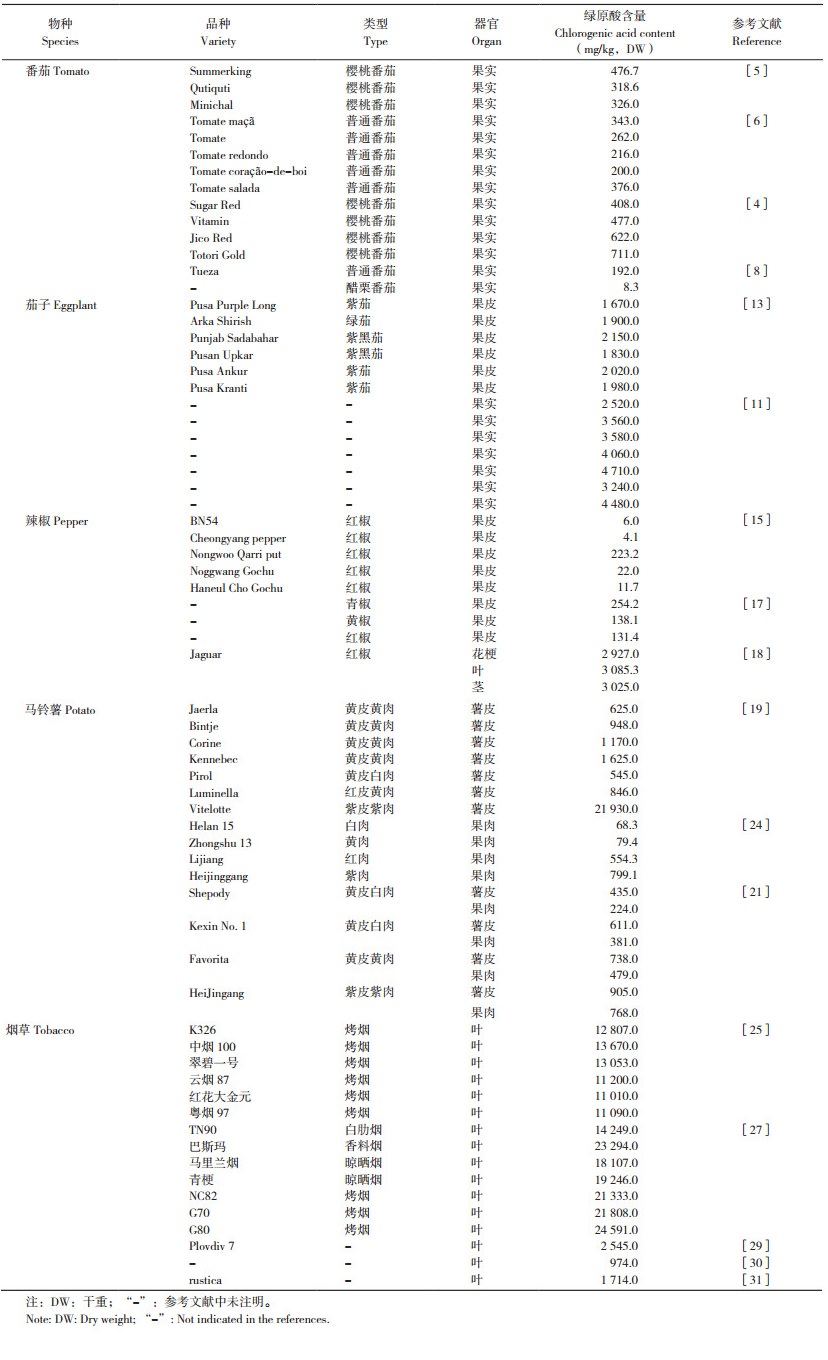
1 5种茄科作物中绿原酸的含量差异绿原酸是番茄、茄子、辣椒、马铃薯、烟草等茄科作物中重要的功能成分,其含量因作物类别或组织的不同而存在较大差异(表 1)。
1.1 番茄中的绿原酸
番茄(Solanum lycopersicum)是世界上最受欢迎的蔬菜之一,其果实中含量最高的酚类物质是柚皮素查尔酮,其次是绿原酸和芦丁,Choi等[4]在12个樱桃番茄品种中测得绿原酸含量为163~711 mg/kg(干重,Dry weight,DW),占所测酚类物质总含量的6.9%~56.3%。Ahn[5]检测了3个樱桃番茄品种中的酚类物质,其中绿原酸含量为318.6~476.7 mg/kg(DW),只占所测酚类物质总量的14.4%~22.1%。Pinela等[6]的结果显示,在18个番茄地方品种中,绿原酸占酚类物质的10.4%~37.7%。以上结果说明番茄中的绿原酸含量低于柚皮素查尔酮,而且不同番茄品种中绿原酸含量差异较大。然而在相同条件下生长的不同番茄品种,其绿原酸含量也有较大差异,为8.1~56.0 mg/kg(鲜重,Fresh weight,FW)[7]。番茄栽培品种Tueza和野生种Solanum pimpinellifolium(LA 1589)中绿原酸含量也呈现出显著差异,其中Tueza的平均含量为192 mg/kg,而LA 1589仅为8.3 mg/kg [8]。
1.2 茄子中的绿原酸茄子富含多酚类物质,而酚酸是茄子中主要的多酚。在茄子不同类型酚酸中,绿原酸是最主要的类型,占茄子总酚酸的70%~90% [9]。Frond等[10]的结果显示,茄子中酚类物质的含量为767 mg/kg(FW),其中酚酸类物质含量最高,并且以绿原酸为主,为621 mg/kg。Rosa- Martínez等[7]也指出,10个茄子品种中的绿原酸平均含量为1 813.9 mg/kg(FW),咖啡酸为34.55 mg/kg(FW),然而所测的其他酚类物质均低于检测限。以上结果表明,绿原酸是茄子中最主要的酚类物质。然而栽培茄子中绿原酸含量较低,平均为2 520 mg/kg(DW),而野生亲缘种茄子的绿原酸较高,平均为3 250 mg/kg,通过鉴定栽培茄子及其野生亲缘茄子果实总酚和绿原酸的含量,可用于培育富含多酚的茄子品种[11]。此外,不同颜色的茄子,其绿原酸含量也有显著差异,如黑茄子和紫茄子均比白茄子的含量高;而同一颜色的茄子果实,其不同部位的含量也有较大差异,即果皮中的绿原酸含量显著高于果肉[12]。Yadav等[13]的研究也指出,在6种重要的茄子品种中,其果皮含有较高含量的绿原酸,为1 670~2 150 mg/kg(DW),这为更好地利用茄子果皮提供了依据。
1.3 辣椒中的绿原酸辣椒在未成熟和成熟时期均可食用,其维生素C含量远高于番茄和茄子。辣椒中多酚类物质种类较多,以黄酮类为主,同时也含有绿原酸[14]。Yi等[15]检测了15个红辣椒品种中的5种酚酸,结果显示有10个品种含量最多的酚酸均是绿原酸,且有7个品种的绿原酸占总酚酸的50% 以上,最高可达90.5%,表明绿原酸也是辣椒中的重要酚酸;而且与成熟果实相比,未成熟果实中绿原酸含量较高。与番茄类似,不同辣椒品种在相同条件下生长时绿原酸的差异也较大,含量为6.0~67.5 mg/kg(FW),变异系数达117.8%[7]。研究表明,不同颜色辣椒的绿原酸也有较大差异。如Fratianni等[16]指出意大利坎帕尼亚地区的黄、红、绿3种传统甜椒品种中的酚酸以没食子酸和绿原酸最多,其中绿原酸含量最高的为绿色品种Friariello Nocerese,含量最低的为黄色品种Cornetto di Acerra,前者含量是后者的9倍。该结果与前人研究墨西哥不同甜椒品种时的趋势一致,即绿原酸在绿色品种中含量较高,从红色品种开始逐渐减少,然后是橙色和黄色品种[16]。Salamatullah等[17]也发现,青椒样品中绿原酸的含量高于黄椒和红椒。目前,也可见到关于辣椒副产物如花梗、叶和茎中多酚含量的报道。如Chel-Guerrero等[18]指出Habanero辣椒花梗、叶和茎中含量较高的多酚是槲皮素、木犀草素、杨梅素和绿原酸,其中绿原酸含量最高的是叶,其次是茎和花梗。表明辣椒副产物对开发用于制药和食品应用的抗炎药物具有潜在的价值。
1.4 马铃薯中的绿原酸马铃薯是世界上重要的粮食作物,其含有人体必需的氨基酸、维生素、矿物质和抗氧化剂等成分,是人们喜爱的健康食品。研究表明,绿原酸是马铃薯主要的多酚类物质[19],其含量占块茎总酚含量的90%[20],在马铃薯皮和果肉中均占主导地位[21]。与果肉相比,马铃薯皮中含有更多的绿原酸,绿原酸含量由薯皮、外果肉至内果肉呈下降趋势,而且品种间存在差异,有的品种薯皮含量高达21 930 mg/kg(DW),而多数品种的含量不超过1 625 mg/kg[19]。杨慧芹等[22]的结果与之类似,在45份马铃薯皮中的绿原酸含量最高为8 579.45 mg/kg,最低为2 422.70 mg/ kg,差异较大。也有研究比较了马铃薯块茎(不去皮)、薯皮和果肉中多酚的差异,结果显示绿原酸的含量从高到低依次为薯皮>块茎>果肉[23]。虽然果肉中绿原酸的含量较低,但果肉颜色不同时,绿原酸也有较大差异,其中,紫肉和红肉品种的绿原酸,极显著高于黄肉和白肉品种[24]。Makori等[21]的研究也证实,不同颜色的马铃薯果肉,绿原酸含量有显著差异,从高到低依次为紫肉>黄肉>白肉。以上研究表明,通常被当作副产物而丢弃的马铃薯皮,可作为功能性食品生产的重要原料,以满足消费者和食品工业的需要。
1.5 烟草中的绿原酸在烟草植株中,绿原酸主要分布于叶片,茎和根中含量较低,绿原酸占烟草多酚总量的75%~90%。不同产地烤烟样品中多酚的含量均以绿原酸最高[25]。在大田生长的成熟烟株中,不同叶位的绿原酸含量有显著差异,其中上部叶最高,其次是中部叶,下部叶最低。即使都是中部叶,其绿原酸在不同烟草品种中也有显著差异[26]。此外,不同类型烟草中绿原酸差异也较大,其中烤烟类与香料烟类品种的含量最高、占2.3%,其次是晾晒烟,白肋烟最低、占1.4%[27]。而同为上部叶,不同产地烟叶中的绿原酸也有较大差异,含量范围为12 520~21 750 mg/kg[28]。此外,在烟草整个生育期中,不同品种烟叶中绿原酸变化趋势也不相同,呈“上升”“上升-下降-上升“M”和“W”型等4种趋势[27]。目前,对于烟草的利用多是将优质烟叶制作卷烟,然而打顶时去掉的顶部枝叶,采收后剩下的茎、根、不适用烟叶,以及卷烟工业的下脚料等通常被当作废弃物,但它们也含有绿原酸、茄尼醇、烟碱等活性物质,这些废弃物的数量巨大,若直接舍弃,不仅浪费资源,还会污染环境,因此可作为提取这些活性物质的重要原料,从而实现环境保护和经济效益的双重收益。
综上,在茄科作物中,绿原酸均是重要的多酚类物质,其在烟草和茄子中含量较高,其次是马铃薯,在番茄和辣椒食用部位中含量稍低。茄子果皮,辣椒花梗、叶和茎等副产物,以及马铃薯皮等,通常被丢弃,然而它们也是绿原酸的重要来源,可作为制药、功能性食品生产、化工等领域的重要原料,因此需要加强其开发利用。
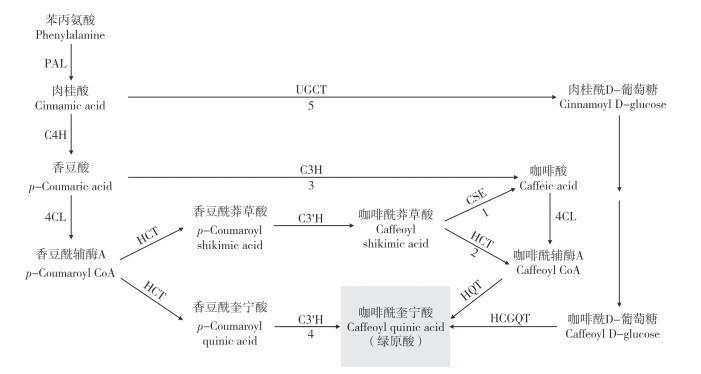
2 绿原酸的生物合成与调控机制 2.1 绿原酸的生物合成途径绿原酸是由咖啡酸与奎宁酸缩合生成的酚酸类化合物。研究表明,绿原酸主要在植物细胞质和叶绿体中合成,然后转移到液泡中储存[32]。绿原酸的生物合成源于苯丙烷代谢,即苯丙氨酸在苯丙氨酸解氨酶(Phenylalanine ammonia-lyase,PAL)的作用下脱氨形成肉桂酸。从肉桂酸开始,有5条已报道的合成途径,如图 1所示。
途径1是肉桂酸在肉桂酸-4-羟化酶(Cinnamic acid 4-hydroxylase,C4H)催化下生成香豆酸(p-Coumaric acid),香豆酸在4- 香豆酸CoA连接酶(4-Coumarate-CoA ligase,4CL)的作用下生成香豆酰辅酶A。然后羟基肉桂酰CoA莽草酸/ 奎宁酸羟基肉桂酰转移酶(Hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase,HCT)催化香豆酰辅酶A与莽草酸反应生成香豆酰莽草酸,香豆酰莽草酸/ 奎宁酸-3′- 羟化酶(p-Coumaroyl-shikimic acid/quinic acid 3′-hydroxylase,C3′H)催化香豆酰莽草酸生成咖啡酰莽草酸,接着咖啡酰莽草酸酯酶(Caffeoyl shikimate esterase,CSE) 水解咖啡酰莽草酸,生成咖啡酸和莽草酸[33],然后咖啡酸通过4CL转化为咖啡酰辅酶A,最后通过羟基肉桂酰CoA:奎宁酸羟基肉桂酰转移酶(Hydroxycinnamoyl -CoA:quinate hydroxycinnamoyl transferase,HQT)与奎宁酸进行酯交换生成绿原酸。研究表明,多数植物通过途径1合成绿原酸[34],该途径中CSE在拟南芥、苜蓿、杨树等被子植物和落叶松等裸子植物咖啡酰莽草酸的转化中发挥了重要作用[33, 35-37]。
途径2是咖啡酰莽草酸在HCT的催化下生成咖啡酰辅酶A,然后由HQT催化奎宁酸与咖啡酰辅酶A生成绿原酸。在番茄和烟草中,HQT在催化咖啡酰辅酶A和奎宁酸生成绿原酸中发挥主要作用[38]。HQT基因的表达水平与马铃薯[20, 39]和其他植物的绿原酸水平相关,如金银花[40]、蒲公英[41]和朝鲜蓟[42]等。Li等[32]也证实HQT的时空表达模式与金银花花中绿原酸积累和分布的动态变化直接相关。Gramazio等[9]对参与茄子绿原酸生物合成的候选基因进行了定位,包括PAL、C4H、4CL、HCT、C3′H和HQT等。Li等[43]通过组装高质量的茄子基因组,综合鉴定了参与绿原酸合成的基因,指出HQT和HCT在茄子、番茄和马铃薯中均由单拷贝基因编码,而拟南芥和辣椒中不存在HQT。以上结果表明,该途径是番茄、茄子、马铃薯和烟草等茄科作物合成绿原酸的主要途径。
途径3中绿原酸是通过咖啡酸生成的,香豆酸在香豆酸-3- 羟化酶(p-coumaric acid 3-hydroxylase,C3H)催化下直接生成咖啡酸。在一些植物如二穗短柄草和玉米中,均没有CSE基因[35],而C3H是最近在二穗短柄草中发现的一种新型过氧化物酶[44],该酶在木质素生物合成的早期步骤中发挥作用。因此,没有CSE基因的植物可以通过途径2或途径3产生绿原酸。
途径4是香豆酰辅酶A在HCT催化下与奎宁酸反应生成香豆酰奎宁酸,再经C3′H羟基化直接生成绿原酸。在拟南芥中,虽然HCT和C3′H均有活性,但并不积累绿原酸,因此,该途径不是大多数植物中绿原酸合成的主要途径。[38]
途径5是从肉桂酸开始,通过UDP- 葡萄糖:肉桂酸葡萄糖转移酶(UDP-glucose:cinnamic acid glucosyltransferase,UGCT) 转化为肉桂酰D-葡萄糖。在甘薯根中发现以咖啡酰D- 葡萄糖作为绿原酸生物合成途径的活性中间体,由羟基肉桂酰-D- 葡萄糖:奎宁酸羟基肉桂酰转移酶(Hydroxycinnamoyl D - glucose:quinate hydroxycinnamoyl transferase,HCGQT)催化咖啡酰D- 葡萄糖和奎宁酸生成绿原酸[34]。
综上,绿原酸合成途径因植物种类而异,在多数茄科作物中主要是途径2。而且绿原酸合成的主要途径最有可能取决于植物特定组织中咖啡酰辅酶A和香豆酰辅酶A相对库容的大小[38, 45]。
2.2 绿原酸生物合成的调控机制绿原酸源于苯丙烷代谢,而植物苯丙烷代谢主要包括苯丙氨酸代谢及下游分支花青素、绿原酸、木质素、芦丁等次生代谢产物的合成[46],其中,绿原酸与木质素共享前体物质香豆酸和香豆酰辅酶A。因绿原酸有益健康,人们对控制它在植物中的生物合成产生了很大兴趣,其研究主要集中在调控催化目标化合物合成的关键酶基因或下游的转录因子方面,如MYB、WRKY、bHLH类等(表 2)。

|
2.2.1 MYB类转录因子对绿原酸积累的调控作用 MYB是真核生物中数量众多、功能多样的转录因子家族之一,在植物苯丙烷代谢调控中发挥重要作用。近年的研究多为拟南芥MYB类转录因子在番茄、马铃薯、烟草等茄科作物中的应用。如拟南芥AtMYB12转录因子,是一种黄酮醇特异性转录激活因子,其在烟草中异位表达时,可导致黄酮醇的高水平积累。将AtMYB12基因在番茄中表达,转基因果实中绿原酸和黄酮醇的含量分别是对照的22、65倍;参与绿原酸合成的C3'H、HCT和HQT基因的转录水平,分别是对照的89.6、49.4和7.83倍[47]。Pandey等[48]的结果与之类似,转AtMYB12基因番茄叶片和果实中黄酮醇和绿原酸积累增加,与黄酮醇生物合成有关的苯丙烷途径基因表达增加。转AtMYB12基因马铃薯块茎绿原酸含量与对照相比明显提高,AtMYB12在马铃薯中激活了苯丙烷代谢途径,FLS、DFR、HQT基因的表达量分别是对照的23、17、60倍[49]。AtMYB111通过特异性激活黄酮醇合成相关基因PAL、CHS、CHI、F3H、FLS的表达,促进烟草黄酮醇和绿原酸的合成,而未引起绿原酸合成相关基因如HQT和HCT转录水平的显著变化[50]。AtMYB11基因的组成型表达增强了PAL、4CL、CHS、F3H、FLS、GT、HCT、HQT等苯丙烷途径基因的表达,导致烟草和番茄植株中黄酮类化合物和绿原酸的积累[51]。AtMYB11在烟草中的调节作用与AtMYB12和AtMYB111相比效率较低[52]。
在咖啡中,CcMYB1抑制苯丙烷途径,其过表达可产生与一般苯丙烷代谢抑制相关的显著表型;CaMYB12和CcMYB12上调苯丙烷途径,导致山奈酚苷、芦丁和绿原酸的较高积累;CcMYB12b通过直接上调HQT基因的表达,可使绿原酸积累水平升高[53]。在灰毡毛忍冬中,LmMYB15与绿原酸含量显著相关,它可以结合并激活4CL、MYB3和MYB4基因的启动子,从而促进绿原酸的合成和苯丙烷代谢[54]。Xu等[55]在甘薯中鉴定到42个转录因子,包括MYBs、bHLHs、ZFPs、bZIPs、ERFs、GARSs、MADSs、WRKY和WD40等,这些转录因子与编码绿原酸生物合成酶的基因有相似的表达模式,推测它们可能参与甘薯绿原酸的调控。
在茄科作物中也鉴定了一些MYB类转录因子,参与调控苯丙烷途径次生代谢产物的合成。如Li等[43]在茄子中鉴定出112个MYB转录因子,包括SG3、SG4、SG6和SG7亚组基因,其中SG4和SG7亚组已被报道可调节苯丙烷途径,包括花青素和黄酮醇的生物合成。茄子SmMyb1转录因子可诱导HQT、CHS、ANS,尤其是DFR基因的表达,从而调节烟草绿原酸的积累和花青素的合成[56]。马铃薯MYB转录因子StAN1(花青素1)在烟草中瞬时表达,诱导了PAL1、PAL2、CHS、F3H、DFR、bHLH1等基因的表达,提高了绿原酸和花青素的含量[57]。在烟草中,NtMYB1、NtMYB2和NtMYB4基因的过表达均可抑制苯丙烷和类黄酮合成途径产物总黄酮、咖啡酸、绿原酸以及芦丁的积累,它们可能通过调控bHLH类转录因子的表达,负调控烟草类黄酮合成途径[58];NtMYB59的过表达可显著提高烟草叶片中的绿原酸含量[59]。
2.2.2 其他类转录因子对绿原酸积累的调控作用 除了MYB类转录因子,其他类如WRKY、bHLH,也参与调控绿原酸的合成以及苯丙烷途径。在蒲公英中,TaWRKY14的过表达使转基因株系具有较高的绿原酸含量和TaPAL1表达量,是因其可与TaPAL1基因启动子的W-box元件相结合[60]。Wang等[61]克隆了2个NtWRKY41基因,发现NtWRKY41a在烟草根、茎、叶、花、腋芽和种子等组织中的表达量均高于NtWRKY41b,过表达NtWRKY41a可促进烟草中绿原酸和木质素的生物合成,抑制莨菪亭和黄酮类化合物的积累;此外,还发现苯丙烷相关差异表达基因NtCCoAOMT和NtHST的转录被显著诱导,而NtF6′H1和NtGT3被显著抑制,并进一步证实NtWRKY41a可与这4个基因的启动子区结合,从而调控它们的转录。最近,他们又鉴定了2个NtWRKY33基因,发现NtWRKY33a可直接与NtMYB4和NtHCT基因的启动子结合,从而抑制芦丁、东莨菪碱和总多酚的积累,同时促进绿原酸的生物合成[62]。此外,Liu等[63]研究发现,TabHLH1基因过表达可上调TaHQT2、Ta4CL、TaCHI和TaF3'H基因的表达,从而显著影响绿原酸和木犀草素的含量,并进一步证实TabHLH1可直接与TaHQT2和Ta4CL基因启动子的bHLH-binding基序结合。
以上研究涉及MYB、WRKY、bHLH类转录因子对绿原酸生物合成及苯丙烷途径的调节,但是目前的研究还不够深入,需要进一步加强对绿原酸生物合成转录调控分子机制以及调控网络的研究。
3 茄科作物绿原酸含量的影响因素绿原酸是茄科作物中重要的酚酸类物质,具有很高的抗氧化活性,药理研究已经证明它们在清除有害自由基和预防疾病方面具有重要的作用。人们通过饮食摄入绿原酸等活性物质,从而使自身获得对抗有害自由基的有效保护剂,维持自身的身体健康。因此,绿原酸含量的多少是影响人们获取这类物质的重要因素。近年来的研究显示,绿原酸含量受基因型和生态环境的影响较大,同时也受栽培措施因素的影响,这些因素决定了茄科作物采收前的绿原酸含量。
3.1 基因型差异对绿原酸含量的影响多数研究表明,基因型是影响茄科作物绿原酸等多酚类物质的重要因素。Martí等[64]指出,除阿魏酸和香豆酸外,基因型对番茄其他多酚的积累有重要影响,其中Kalvert番茄对包括绿原酸、咖啡酸、芦丁、杨梅酮、槲皮素、柚皮素在内的大多数多酚的积累明显突出。Çolak等[8]检测了番茄栽培品种Tueza和野生种S. pimpinellifolium(LA 1589)以及LA 1589种间回交自交系群体的酚酸含量,其中Tueza的绿原酸是LA 1589的23.13倍,而回交自交系群体的平均含量与LA 1589的接近。茄子栽培品种及其野生亲缘种果实的绿原酸含量也有明显差异,其中野生亲缘种的平均含量是栽培茄子的1.29倍[11]。在11份烤烟和6份晒烟种质资源成熟期叶片中,绿原酸含量高于1 000 mg/kg的种质有9份,其中烤烟5份,晒烟4份,含量较高的种质中以晒烟所占的比例较大[65]。二倍体马铃薯中绿原酸水平的最显著决定因素也是基因型,如在祖先种类群S. candolleanum,地方种类群S. stenotomum、S. goniocalyx和S. phureja中,其块茎果肉绿原酸水平的变化分别为2.80、9.19、1.87、7.23倍[66]。Suo等[67]检测了不同基因型马铃薯果肉中酚类物质的含量,其中绿原酸最高值是最低值的13.86倍。二倍体马铃薯皮中绿原酸含量也因基因型差异有较大差别,在3个亚种共45份材料中,最高含量的是最低含量的3.54倍[22]。在同一地点种植的25个烟草品种中,其绿原酸含量相差6.22倍[68]。在药用植物金银花中,绿原酸的水平也因基因型差异有较大不同,在金银花的各生育期,四倍体品种的绿原酸均显著高于二倍体[69-70]。
3.2 环境因素对绿原酸含量的影响除了基因型的差异,温度、光照、水分、海拔等生长环境因素对茄科作物绿原酸等多酚类物质也有较大影响。如在西班牙西南部Extremadura种植的番茄与在西班牙东北部Navarra种植的番茄相比,其所含绿原酸和阿魏酸较高,芦丁较低,其他多酚在两个地点的含量相似,这种差异是由Extremadura较高的温度和光照水平引起的[64]。Fibiani等[71]的结果显示,采收前的气候条件不同,番茄果实绿原酸等活性物质也有显著差异,其中2016年(采收前低温多雨)和2017年(采收前高温少雨)的绿原酸含量与2015年(采收前高温)相比显著提高。Nwanna等[72]指出,环境因素对茄子酚类物质的总含量有显著影响,其中绿原酸、没食子酸、表儿茶素、芦丁等大多数酚类物质在Uyo种植时的含量显著高于Ibadan的,他们通过进一步分析发现这种差异是因两个地点温度的不同而产生的。Gutiérrez-Quequezana等[73]研究表明,在13 ℃条件下生长的Blue Congo和Blaue Veltlin马铃薯品种,其块茎的绿原酸含量比18 ℃条件下的分别高13% 和18%。将25个烟草品种在4个气候条件不同的地方种植时,绿原酸含量也有较大差异,其中ERB-26品种在Karayaka种植时是在Gümüşhacıköy的12.44倍[68]。Piñeros-Niño等[74]评估了2种气候条件对马铃薯酚类物质的影响,发现在Usme(海拔3 400 m)种植的其绿原酸平均值是在Facatativá(海拔2 650 m)的2倍。在其他植物如莴苣中,连续光照、蓝色LED处理、CO2浓度升高以及昼长增加等条件下,均可促进绿原酸的积累[75]。在多年生常绿植物阿拉比卡咖啡中,自然光照12 h/12 h周期下叶片中绿原酸的积累水平,显著高于生长在短光照8 h/16 h下的,说明绿原酸的产生受光周期的影响[76]。
3.3 栽培措施对绿原酸含量的影响在基因型和环境因素条件不变的情况下,不同的栽培模式、矿质元素、灌溉方式及农艺措施等对绿原酸的积累也有重要作用。Hallmann[77]在比较有机栽培和常规栽培模式下番茄多酚类物质积累差异时指出,有机栽培模式下生长的番茄其绿原酸含量显著高于常规栽培的,而栽培模式对没食子酸无显著影响。Fibiani等[71]的结果与之相似,即有机且自然覆盖栽培模式与常规栽培模式相比,番茄果实的绿原酸含量显著提高。在茄子中,有机栽培和常规栽培模式下的绿原酸含量差异不显著[78]。而在甜椒果实中,有机栽培条件下的绿原酸含量也显著高于常规栽培的[79]。Camacho-Cristóbal等[80]分析了硼元素缺乏对烟草叶片的影响,结果发现与对照组相比,硼缺乏条件下烟草叶片碳水化合物和总酚类物质显著增加,其中包括绿原酸水平显著增加。Flores等[81]在研究土壤元素组成与番茄中生物活性物质的关系时发现,土壤中钼、镍含量较高时,果实中绿原酸和槲皮素的含量较高。Kurt等[82]比较了灌溉、氮素形态和留叶数对晒烟产量和品质性状的影响,结果显示,晒烟的绿原酸随着水分胁迫的增加而增加,其中非灌溉处理时绿原酸含量最高;在施硝酸铵的情况下,绿原酸含量显著高于施其他氮素形态的肥料;绿原酸的含量与留叶数成反比,即23片/株 > 28片/株的。
4 展望绿原酸是一种天然的酚酸类化合物,具有多种生物功能,广泛存在于茄科作物中,其中在烟草和茄子中含量较高,其次是马铃薯,在番茄和辣椒食用部位中含量稍低,其含量的高低受基因型的影响较大,同时也因环境条件和栽培措施等的差异而不同。绿原酸在功能性食品、营养补充剂、食品原料、化工和新药开发等方面具有很大潜力,而茄子果皮,辣椒花梗、叶片和茎,马铃薯皮,烟草打顶时去掉的顶部枝叶,采收时剩下的茎及不适用烟叶,以及卷烟工业的下脚料等副产物,也是绿原酸的重要来源,若将其直接舍弃则会造成资源的极大浪费,因此,对于这些副产物,需要进一步加强其开发利用。
目前,绿原酸的生物合成途径以及参与合成的各种结构基因已较为清晰,但仍需进一步克隆并利用这些关键基因,绿原酸的转录调控机制也取得了一些进展,然而多是拟南芥转录因子在茄科作物中的应用,以及蒲公英、忍冬等药用植物转录因子与结构基因的互作关系,尚缺乏茄科作物绿原酸转录调控机制以及调控网络的研究。除了MYB、WRKY、bHLH类已报道的这些转录因子,还有哪些转录因子调控绿原酸的生物合成?这些转录因子响应何种生物或非生物胁迫?它们与结构基因的互作关系是怎样的?其是否还能与其他转录因子结合形成复合物从而共同调控绿原酸的生物合成?为了解答这些问题,需要进一步挖掘茄科作物绿原酸合成的转录调控因子,解析其调控机制及调控网络,以期为高绿原酸茄科作物种质资源创制及新品种的培育工作提供理论指导。
| [1] |
王庆华, 杜婷婷, 张智慧, 季鸣, 胡海宇, 陈晓光. 绿原酸的药理作用及机制研究进展[J]. 药学学报, 2020, 55(10): 2273-2280. DOI:10.16438/j.0513-4870.2020-0423 WANG Q H, DU T T, ZHANG Z H, JI M, HU H Y, CHEN X G. Advances in research on the pharmacological effects and mechanism of action of chlorogenic acid[J]. Acta Pharmaceutica Sinica, 2020, 55(10): 2273-2280. DOI:10.16438/j.0513-4870.2020-0423 |
| [2] |
HODAEI M, RAHIMMALEK M, BEHBAHANI M. Anticancer drug discovery from Iranian Chrysanthemum cultivars through system pharmacology exploration and experimental validation[J]. Scientifi c Reports, 2021, 11: 11767. DOI:10.1038/s41598-021-91010-y |
| [3] |
SANTANA-GÁLVEZ J, CISNEROS-ZEVALLOS L, JACOBO-VELÁZQUEZ D A. Chlorogenic acid: recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome[J]. Molecules, 2017, 22(3): 358. DOI:10.3390/molecules22030358 |
| [4] |
CHOI S H, KIM D-S, KOZUKUE N, KIM H-J, NISHITANI Y, MIZUNO M, LEVIN C E, FRIEDMAN M. Protein, free amino acid, phenolic, β-carotene, and lycopene content, and antioxidative and cancer cell inhibitory effects of 12 greenhouse-grown commercial cherry tomato varieties[J]. Journal of Food Composition and Analysis, 2014, 34: 115-127. DOI:10.1016/j.jfca.2014.03.005 |
| [5] |
AHN J B. Characterization of lycopene, β-carotene, and phenolic compounds of domestic cherry tomato cultivars[J]. Food Engineering Progress, 2018, 22(1): 9-16. DOI:10.13050/foodengprog.2018.22.1.9 |
| [6] |
PINELA J, MONTOYA C, CARVALHO A M, MARTINS V, ROCHA F, BARATA A M, BARROS L, FERREIRA I C F R. Phenolic composition and antioxidant properties of ex-situ conserved tomato (Solanum lycopersicum L.) germplasm[J]. Food Research International, 2019, 125: 108545. DOI:10.1016/j.foodres.2019.108545 |
| [7] |
ROSA-MARTÍNEZ E, GARCÍA-MARTÍNEZ M D, ADALID-MARTÍNEZ A M, PEREIRA-DIAS L, CASANOVA C, SOLER E, FIGÀS M R, RAIGÓN M D, PLAZAS M, SOLER S, PROHENS J. Fruit composition profile of pepper, tomato and eggplant varieties grown under uniform conditions[J]. Food Research International, 2021, 147: 110531. DOI:10.1016/j.foodres.2021.110531 |
| [8] |
ÇOLAK N G, EKEN N T, ÜLGER M, FRARY A, DOĞANLAR S. Mapping of quantitative trait loci for antioxidant molecules in tomato fruit: carotenoids, vitamins C and E, glutathione and phenolic acids[J]. Plant Science, 2020, 292: 110393. DOI:10.1016/j.plantsci.2019.110393 |
| [9] |
GRAMAZIO P, PROHENS J, PLAZAS M, ANDJAR I, HERRAIZ F J, CASTILLO E, KNAPP S, MEYER R S, VILANOVA S. Location of chlorogenic acid biosynthesis pathway and polyphenol oxidase genes in a new interspecific anchored linkage map of eggplant[J]. BMC Plant Biology, 2014, 14: 350. DOI:10.1186/s12870-014-0350-z |
| [10] |
FROND A D, IUHAS C I, STIRBU I, LEOPOLD L, SOCACI S, ANDREEA S, AYVAZ H, ANDREEA S, MIHAI S, DIACONEASA Z, CARMEN S. Phytochemical characterization of five edible purple-reddish vegetables: anthocyanins, flavonoids, and phenolic acid derivatives[J]. Molecules, 2019, 24: 1536. DOI:10.3390/molecules24081536 |
| [11] |
KAUSHIK P. Characterization of cultivated eggplant and its wild relatives based on important fruit biochemical traits[J]. Pakistan Journal of Biological Sciences, 2020, 23: 1220-1226. DOI:10.3923/pjbs.2020.1220.1226 |
| [12] |
COLAK N, KURT-CELEBI A, GRUZ J, STRNAD M, HAYIRLIOGLU-AYAZ S, CHOUNG M-G, ESATBEYOGLU T, AYAZ F A. The phenolics and antioxidant properties of black and purple versus white eggplant cultivars[J]. Molecules, 2022, 27: 2410. DOI:10.3390/molecules27082410 |
| [13] |
YADAV V K, SINGH R, JHA R K, KAUSHIK P. Biochemical variability of eggplant peel among Indian cultivars[J]. Indian Journal of Biochemistry & Biophysics, 2020, 57: 634-637. |
| [14] |
CALUMPANG C L F, SAIGO T, WATANABE M, TOHGE T. Cross-species comparison of fruit-metabolomics to elucidate metabolic regulation of fruit polyphenolics among Solanaceous crops[J]. Metabolites, 2020, 10: 209. DOI:10.3390/metabo10050209 |
| [15] |
YI T G, PARK Y, CHOI I-Y, PARK N-I. Comparison of metabolite levels and antioxidant activity among pepper cultivars[J]. Korean Journal of Breeding Science, 2019, 51(4): 326-340. DOI:10.9787/KJBS.2019.51.4.326 |
| [16] |
FRATIANNI F, ACIERNO A Dˊ, COZZOLINO A, SPIGNO P, RICCARDI R, RAIMO F, PANE C, ZACCARDELLI M, LOMBARDO V T, TUCCI M, GRILLO S, COPPOLA R, NAZZAR F. Biochemical characterization of traditional varieties of sweet pepper (Capsicum annuum L.) of the Campania region, southern Italy[J]. Antioxidants, 2020, 9: 556. DOI:10.3390/antiox9060556 |
| [17] |
SALAMATULLAH A M, HAYAT K, HUSAIN F M, AHMED M A, ARZOO S, ALTHBITI M M, ALZAHRANI A, AL-ZAIED B A M, ALYAHYA H K, ALBADER N, NAFIDI H-A, BOURHIA M. Effects of different solvents extractions on total polyphenol content, HPLC analysis, antioxidant capacity, and antimicrobial properties of peppers (red, yellow, and green (Capsicum annum L.)[J]. Evidence-Based Complementary and Alternative Medicine, 2022, 7372101. DOI:10.1155/2022/7372101 |
| [18] |
CHEL-GUERRERO L D, CASTAÑEDA-CORRAL G, LÓPEZ-CASTILLO M, SCAMPICCHIO M, MOROZOVA K, ONEY-MONTALVO J E, FERRENTINO G, ACEVEDO-FERNÁNDEZ J J, RODRÍGUEZ-BUENFIL I M. In vivo anti-inflammatory effect, antioxidant activity, and polyphenolic content of extracts from Capsicum chinense by-products[J]. Molecules, 2022, 27: 1323. DOI:10.3390/molecules27041323 |
| [19] |
DEUßER H, GUIGNARD C, HOFFMANN L, EVERS D. Polyphenol and glycoalkaloid contents in potato cultivars grown in Luxembourg[J]. Food Chemistry, 2012, 135: 2814-2824. DOI:10.1016/j.foodchem.2012.07.028 |
| [20] |
PAYYAVULA R S, SHAKYA R, SENGODA V G, MUNYANEZA J E, SWAMY P, NAVARRE D A. Synthesis and regulation of chlorogenic acid in potato: rerouting phenylpropanoid flux in HQT-silenced lines[J]. Plant Biotechnology Journal, 2015, 13: 551-564. DOI:10.1111/pbi.12280 |
| [21] |
MAKORI S I, MU T H, SUN H N. Profiling of polyphenols, flavonoids and anthocyanins in potato peel and flesh from four potato varieties[J]. Potato Research, 2022, 65: 193-208. DOI:10.1007/s11540-021-09516-x |
| [22] |
杨慧芹, 张丽颖, 余万都, 尚轶, 马玲. 二倍体马铃薯皮绿原酸含量的研究[J]. 云南民族大学学报: 自然科学版, 2021, 30(2): 125-129. DOI:10.3969/j.issn.1672-8513.2021.02.005 YANG H Q, ZHANG L Y, YU W D, SHANG Y, MA L. Study on the content of chlorogenic acid in diploid potato skin[J]. Journal of Yunnan Minzu University: Natural Sciences Edition, 2021, 30(2): 125-129. DOI:10.3969/j.issn.1672-8513.2021.02.005 |
| [23] |
KIM J, SOH S Y, BAE H, NAM S-Y. Antioxidant and phenolic contents in potatoes (Solanum tuberosum L.) and micropropagated potatoes[J]. Applied Biological Chemistry, 2019, 62: 17. DOI:10.1186/s13765-019-0422-8 |
| [24] |
RU W D, PANG Y H, GAN Y R, LIU Q, BAO J S. Phenolic compounds and antioxidant activities of potato cultivars with white, yellow, red and purple flesh[J]. Antioxidants, 2019, 8: 419. DOI:10.3390/antiox8100419 |
| [25] |
李力, 李东亮, 邓发达, 罗诚, 沈怡, 薛芳. UPLC法同时测定烤烟中6种多酚的研究[J]. 中国农学通报, 2018, 34(10): 131-137. DOI:10.11924/j.issn.1000-6850.casb17030160 LI L, LI D L, DENG F D, LUO C, SHEN Y, XUE F. Analysis of 6 polyphenols in flue-cured tobacco by UPLC[J]. Chinese Agricultural Science Bulletin, 2018, 34(10): 131-137. DOI:10.11924/j.issn.1000-6850.casb17030160 |
| [26] |
TSABALLA A, SARROU E, XANTHOPOULOU A, TSALIKI E, KISSOUDIS C, KARAGIANNIS E, MICHAILIDIS M, MARTENS S, SPERDOULI E, HILIOTI Z, FOTOPOULOS V, NIANIOU-OBEIDAT I, TSAFTARIS A, MADESIS P, KALIVAS A, GANOPOULOS I. Comprehensive approaches reveal key transcripts and metabolites highlighting metabolic diversity among three oriental tobacco varieties[J]. Industrial Crops & Products, 2020, 143: 111933. DOI:10.1016/j.indcrop.2019.111933 |
| [27] |
万诚, 刘仁祥, 聂琼, 龙丽, 徐如宏. 不同烟草品种绿原酸含量变化研究[J]. 山地农业生物学报, 2016, 32(2): 25-28. DOI:10.15958/j.cnki.sdnyswxb.2016.02.005 WAN C, LIU R X, NIE Q, LONG L, XU R H. Dynamic changes of chlorogenic acid content in different types of tobacco cultivars[J]. Journal of Mountain Agriculture and Biology, 2016, 32(2): 25-28. DOI:10.15958/j.cnki.sdnyswxb.2016.02.005 |
| [28] |
史春云, 袁凯龙, 肖卫强, 卢昕博, 戴路. 超高效液相色谱法快速检测烟叶中8种多酚类化合物[J]. 西南农业学报, 2015, 28(3): 1322-1327. DOI:10.16213/j.cnki.scjas.2015.03.075 SHI C Y, YUAN K L, XIAO W Q, LU X B, DAI L. Fast determination of eight kinds of polyphenols in tobacco leaf with UPLC[J]. Southwest China Journal of Agricultural Sciences, 2015, 28(3): 1322-1327. DOI:10.16213/j.cnki.scjas.2015.03.075 |
| [29] |
POPOVA V, IVANOVA T, STOYANOVA A, GEORGIEV V, HRISTEVA T, NIKOLOVA V, DOCHEVA M, NIKOLOV N, DAMYANOVA S. Phytochemicals in leaves and extracts of the variety "Plovdiv 7" of Bulgarian oriental tobacco (Nicotiana tabacum L.)[J]. Trends in Phytochemical Research, 2018, 2(1): 27-36. |
| [30] |
POPOVA V, IVANOVA T, STOYANOVA A, NIKOLOVA V, HRISTEVA T, DOCHEVA M, NIKOLOV N, ILIEV I. Polyphenols and triterpenes in leaves and extracts from three Nicotiana species[J]. Journal of Applied Biology & Biotechnology, 2019, 7(5): 45-49. DOI:10.7324/JABB.2019.70508 |
| [31] |
POPOVA V T, IVANOVA T A, STOYANOVA A S, NIKOLOVA V V, DOCHEVA M H, HRISTEVA T H, DAMYANOVA S T, NIKOLOV N P. Chemical constituents in leaves and aroma products of Nicotiana rustica L. tobacco[J]. International Journal of Food Studies, 2020, 9: 146-159. DOI:10.7455/ijfs/9.1.2020.a2 |
| [32] |
LI Y Q, KONG D X, BAI M, HE H J, WANG H Y, WU H. Correlation of the temporal and spatial expression patterns of HQT with the biosynthesis and accumulation of chlorogenic acid in Lonicera japonica flowers[J]. Horticulture Research, 2019, 6: 73. DOI:10.1038/s41438-019-0154-2 |
| [33] |
VANHOLME R, CESARINO I, RATAJ K, XIAO Y G, SUNDIN L, GOEMINNE G, KIM H, CROSS J, MORREEL K, ARAUJO P, WELSH L, HAUSTRAETE J, MCCLELLAN C, VANHOLME B, RALPH J, SIMPSON G G, HALPIN C, BOERJAN W. Caffeoyl shikimate esterase (CSE) is an enzyme in the lignin biosynthetic pathway in Arabidopsis[J]. Science, 2013, 341: 1103-1106. DOI:10.1126/science.1241602 |
| [34] |
MAGAÑA A A, KAMIMURA N, SOUMYANATH A, STEVENS J F, MAIER C S. Caffeoylquinic acids: chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity[J]. The Plant Journal, 2021, 107: 1299-1319. DOI:10.1111/tpj.15390 |
| [35] |
HA C M, ESCAMILLA-TREVINO L, YARCE J C S, KIM H, RALPH J, CHEN F, DIXON R A. An essential role of caffeoyl shikimate esterase in monolignol biosynthesis in Medicago truncatula[J]. The Plant Journal, 2016, 86: 363-375. DOI:10.1111/tpj.13177 |
| [36] |
SALEME M D L S, CESARINO I, VARGAS L, KIM H, VANHOLME R, GOEMINNE G, ACKER R V, FONSECA F C D A, PALLIDIS A, VOOREND W, JUNIOR J N, PADMAKSHAN D, DOORSSELAERE J V, RALPH J, BOERJAN W. Silencing caffeoyl shikimate esterase affects lignification and improves saccharification in poplar[J]. Plant Physiology, 2017, 175: 1040-1057. DOI:10.1104/pp.17.00920 |
| [37] |
WANG X C, CHAO N, ZHANG M, JIANG X N, GAI Y. Functional characteristics of caffeoyl shikimate esterase in Larix kaempferi and monolignol biosynthesis in gymnosperms[J]. International Journal of Molecular Sciences, 2019, 20: 6071. DOI:10.3390/ijms20236071 |
| [38] |
NIGGEWEG R, MICHAEL A J, MARTIN C. Engineering plants with increased levels of the antioxidant chlorogenic acid[J]. Nature Biotechnology, 2004, 22: 746-754. DOI:10.1038/nbt966 |
| [39] |
VALIÑAS M A, LANTERI M L, HAVE A T, ANDREU A B. Chlorogenic acid, anthocyanin and flavan-3-ol biosynthesis in flesh and skin of Andean potato tubers (Solanum tuberosum subsp. andigena)[J]. Food Chemistry, 2017, 229: 837-846. DOI:10.1016/j.foodchem.2017.02.150 |
| [40] |
ZHANG J R, WU M L, LI W D, BAI G B. Regulation of chlorogenic acid biosynthesis by hydroxycinnamoyl CoA quinate hydroxycinnamoyl transferase in Lonicera japonica[J]. Plant Physiology and Biochemistry, 2017, 121: 74-79. DOI:10.1016/j.plaphy.2017.10.017 |
| [41] |
刘群. 丹东蒲公英绿原酸生物合成关键酶TaHQTs调控研究[D]. 沈阳: 沈阳农业大学, 2019: 33-34. LIU Q. Research on the regulation of CGA biosynthesis key enzyme TaHQTs in Taraxacum antungense Kitag[D]. Shenyang: Shenyang Agricultural University, 2019: 33-34. |
| [42] |
SONNANTE G, D'AMORE R, BLANCO E, PIERRI C L, PALMA M D, LUO J, TUCCI M, MARTIN C. Novel hydroxycinnamoyl-coenzyme A quinate transferase genes from artichoke are involved in the synthesis of chlorogenic acid[J]. Plant Physiology, 2010, 153: 1224-1238. DOI:10.1104/pp.109.150144 |
| [43] |
LI D D, QIAN J, LI W L, YU N, GAN G Y, JIANG Y Q, LI W J, LIANG X Y, CHEN R Y, MO Y C, LIAN J M, NIUY C, WANG Y K. A high-quality genome assembly of the eggplant provides insights into the molecular basis of disease resistance and chlorogenic acid synthesis[J]. Molecular Ecology Resources, 2021, 21: 1274-1286. DOI:10.1111/1755-0998.13321 |
| [44] |
BARROS J, ESCAMILLA-TREVINO L, SONG L H, RAO X L, SERRANI-YARCE J C, PALACIOS M D, ENGLE N, CHOUDHURY F K, TSCHAPLINSKI T J, VENABLES B J, MITTLER R, DIXON R A. 4-Coumarate 3-hydroxylase in the lignin biosynthesis pathway is a cytosolic ascorbate peroxidase[J]. Nature Communications, 2019, 10: 1994. DOI:10.1038/s41467-019-10082-7 |
| [45] |
SILVA N V E, MAZZAFERA P, CESARINO I. Should I stay or should I go: are chlorogenic acids mobilized towards lignin biosynthesis?[J]. Phytochemistry, 2019, 166: 112063. DOI:10.1016/j.phytochem.2019.112063 |
| [46] |
王星, 罗双霞, 于萍, 罗蕾, 赵建军, 王彦华, 申书兴, 陈雪平. 茄科蔬菜苯丙烷类代谢及相关酶基因研究进展[J]. 园艺学报, 2017, 44(9): 1738-1748. DOI:10.16420/j.issn.0513-353x.2017-0499 WANG X, LUO S X, YU P, LUO L, ZHAO J J, WANG Y H, SHEN S X, CHEN X P. Advances in phenylaprapanoid metabolism and its enzyme genes in Solanaceae vegetables[J]. Acta Horticulturae Sinica, 2017, 44(9): 1738-1748. DOI:10.16420/j.issn.0513-353x.2017-0499 |
| [47] |
LUO J, BUTELLI E, HILL L, PARR A, NIGGEWEG R, BAILEY P, WEISSHAAR B, MARTIN C. AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: expression in fruit results in very high levels of both types of polyphenol[J]. The Plant Journal, 2008, 56: 316-326. DOI:10.1111/j.1365-313X.2008.03597.x |
| [48] |
PANDEY A, MISRA P, CHOUDHARY D, YADAV R, GOEL R, BHAMBHANI S, SANYAL I, TRIVEDI R, TRIVEDI P K. AtMYB12 expression in tomato leads to large scale differential modulation in transcriptome and flavonoid content in leaf and fruit tissues[J]. Scientific Reports, 2015, 5: 12412. DOI:10.1038/srep12412 |
| [49] |
代丽丽. 转AtMYB12基因提高马铃薯中绿原酸含量的研究[D]. 泰安: 山东农业大学, 2013: 35-36. DAI L L. Research of chlorogenic acid-improved transgenic potato with AtMYB12[D]. Taian: Shandong Agricultural University, 2013: 35-36. |
| [50] |
PANDEY A, MISRA P, BHAMBHANI S, BHATIA C, TRIVEDI P K. Expression of Arabidopsis MYB transcription factor, AtMYB111, in tobacco requires light to modulate flavonol content[J]. Scientifi c Reports, 2014, 4: 5018. DOI:10.1038/srep05018 |
| [51] |
LI Y, CHEN M, WANG S L, NING J, DING X H, CHU Z H. AtMYB11 regulates caffeoylquinic acid and flavonol synthesis in tomato and tobacco[J]. Plant Cell Tissue and Organ Culture, 2015, 122: 309-319. DOI:10.1007/s11240-015-0767-6 |
| [52] |
PANDEY A, MISRA P, TRIVEDI P K. Constitutive expression of Arabidopsis MYB transcription factor, AtMYB11, in tobacco modulates flavonoid biosynthesis in favor of flavonol accumulation[J]. Plant Cell Reports, 2015, 34: 1515-1528. DOI:10.1007/s00299-015-1803-z |
| [53] |
KNEVITT D. Characterising chlorogenic acid biosynthesis in coffee[D]. Norwich: University of East Anglia, 2016: 152-160.
|
| [54] |
TANG N, CAO Z Y, YANG C, RAN D S, WU P Y, GAO H M, HE N, LIU G H, CHEN Z X. A R2R3-MYB transcriptional activator LmMYB15 regulates chlorogenic acid biosynthesis and phenylpropanoid metabolism in Lonicera macranthoides[J]. Plant Science, 2021, 308: 110924. DOI:10.1016/j.plantsci.2021.110924 |
| [55] |
XU J, ZHU J H, LIN Y H, ZHU H L, TANG L Q, WANG X H, WANG X N. Comparative transcriptome and weighted correlation network analyses reveal candidate genes involved in chlorogenic acid biosynthesis in sweet potato[J]. Scientifi c Reports, 2022, 12: 2770. DOI:10.1038/s41598-022-06794-4 |
| [56] |
DOCIMO T, FRANCESE G, RUGGIERO A, BATELLI G, PALMA M D, BASSOLINO L, TOPPINO L, ROTINO G L, MENNELLA G, TUCCI M. Phenylpropanoids accumulation in eggplant fruit: characterization of biosynthetic genes and regulation by a MYB transcription factor[J]. Frontiers in Plant Science, 2016, 6: 1233. DOI:10.3389/fpls.2015.01233 |
| [57] |
PAYYAVULA R S, SINGH R K, NAVARRE D A. Transcription factors, sucrose, and sucrose metabolic genes interact to regulate potato phenylpropanoid metabolism[J]. Journal of Experimental Botany, 2013, 64(16): 5115-5131. DOI:10.1093/jxb/ert303 |
| [58] |
陈帅. 烟草类黄酮代谢途径中关键酶CHS基因与R2R3 MYB类转录抑制因子功能研究[D]. 成都: 四川农业大学, 2017: 60-65. CHEN S. Functional analysis of CHS genes and R2R3 MYB repressors related to flavonoid biosynthesis pathway in Nicotiana tabacum[D]. Chengdu: Sichuan Agricultural University, 2017: 60-65. |
| [59] |
王中, 赵利杰, 刘萍萍, 郑美, 谢小东, 王晨, 张剑锋, 罗朝鹏, 杨军, 武明珠. 烟草NtMYB59基因克隆及过表达对绿原酸含量的影响[J]. 烟草科技, 2021, 54(5): 1-7. DOI:10.16135/j.issn1002-0861.2020.0288 WANG Z, ZHAO L J, LIU P P, ZHENG M, XIE X D, WANG C, ZHANG J F, LUO C P, YANG J, WU M Z. Cloning and over-expressing tobacco NtMYB59 gene and the effects on chlorogenic acid content[J]. Tobacco Science & Technology, 2021, 54(5): 1-7. DOI:10.16135/j.issn1002-0861.2020.0288 |
| [60] |
LIU Q, ZHOU W, RUAN Q Y, CHENG H T, LIU T Y, WANG L R, YUAN Y, LI L, WU J, JIANG J H, NING W, KAI G Y. Overexpression of TaWRKY14 transcription factor enhances accumulation of chlorogenic acid in Taraxacum antungense Kitag and increases its resistance to powdery mildew[J]. Plant Cell, Tissue and Organ Culture, 2020, 143: 665-679. DOI:10.1007/s11240-020-01950-y |
| [61] |
WANG Z, WANG S B, LIU P P, YANG X N, HE X X, XIE X D, LUO Z P, WU M Z, WANG C, YANG J. Molecular cloning and functional characterization of NtWRKY41a in the biosynthesis of phenylpropanoids in Nicotiana tabacum[J]. Plant Science, 2022, 315: 111154. DOI:10.1016/j.plantsci.2021.111154 |
| [62] |
WANG Z, MA L X, LIU P P, LUO Z P, LI Z F, WU M Z, XU X, PU W X, HUANG P J, YANG J. Transcription factor NtWRKY33a modulates the biosynthesis of polyphenols by targeting NtMYB4 and NtHCT genes in tobacco[J]. Plant Science, 2023, 326: 111522. DOI:10.1016/j.plantsci.2022.111522 |
| [63] |
LIU Q, LI L, CHENG H T, YAO L X, WU J, HUANG H, NING W, KAI G Y. The basic helix-loop-helix transcription factor TabHLH1 increases chlorogenic acid and luteolin biosynthesis in Taraxacum antungense Kitag[J]. Horticulture Research, 2021, 8: 195. DOI:10.1038/s41438-021-00630-y |
| [64] |
MARTÍ R, LEIVA-BRONDO M, LAHOZ I, CAMPILLO C, CEBOLLA-CORNEJO J, ROSELLÓ S. Polyphenol and L-ascorbic acid content in tomato as influenced by high lycopene genotypes and organic farming at different environments[J]. Food Chemistry, 2018, 239: 148-156. DOI:10.1016/j.foodchem.2017.06.102 |
| [65] |
邵秀红, 马柱文, 袁清华, 李集勤, 潘晓英, 黄振瑞. 烟草种质资源重要活性成分含量差异研究[J]. 广东农业科学, 2021, 48(12): 91-99. DOI:10.16768/j.issn.1004-874X.2021.12.011 SHAO X H, MA Z W, YUAN Q H, LI J Q, PAN X Y, HUANG Z R. Study on content differences of important active ingredients in tobacco germplasm resources[J]. Guangdong Agricultural Sciences, 2021, 48(12): 91-99. DOI:10.16768/j.issn.1004-874X.2021.12.011 |
| [66] |
YANG H Q, LIAO Q G, MA L, LUO W, XIONG X F, LUO Y W, YANG X, DU C Y, HE Y F, LI X L, GAO D L, XUE X F, SHANG Y. Features and genetic basis of chlorogenic acid formation in diploid potatoes[J]. Food Chemistry: Molecular Sciences, 2021, 3: 100039. DOI:10.1016/j.fochms.2021.100039 |
| [67] |
SUO H C, PENG Z T, GUO Z Q, WU C J H, LIU J T, WANG L, XIAO J, LI X B. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from different potato genotypes: comparison of free and bound phenolic profiles and antioxidant activity[J]. Food Chemistry, 2022, 388: 133058. DOI:10.1016/j.foodchem.2022.133058 |
| [68] |
KURT D. Impacts of environmental variations on quality and chemical contents of oriental tobacco[J]. Contributions to Tobacco and Nicotine Research, 2021, 30: 50-62. DOI:10.2478/cttr-2021-0006 |
| [69] |
KONG D X, LI Y Q, BAI M, DENG Y L, LIANG G X, WU H. A comparative study of the dynamic accumulation of polyphenol components and the changes in their antioxidant activities in diploid and tetraploid Lonicera japonica[J]. Plant Physiology and Biochemistry, 2017, 112: 87-96. DOI:10.1016/j.plaphy.2016.12.027 |
| [70] |
WANG H L, LI Y Q, WANG S B, KONG D X, SAHU S K, BAI M, LI H Y, LI L Z, XU Y, LIANG H P, LIU H, WU H. Comparative transcriptomic analyses of chlorogenic acid and luteolosides biosynthesis pathways at different flowering stages of diploid and tetraploid Lonicera japonica[J]. PeerJ, 2020, 8: e8690.. DOI:10.7717/peerj.8690 |
| [71] |
FIBIANI M, PAOLO D, LETEO F, CAMPANELLI G, PICCHI V, BIANCHI G, LO SCALZO R. Influence of year, genotype and cultivation system on nutritional values and bioactive compounds in tomato (Solanum lycopersicum L.)[[J]. Food Chemistry, 2022, 389: 133090. DOI:10.1016/j.foodchem.2022.133090 |
| [72] |
NWANNA E E, ADEBAYO A A, ADEMOSUN A O, OBOH G. Phenolic distribution, antioxidant activity, and enzyme inhibitory properties of eggplant (Solanum aethiopicum) cultivated in two different locations within Nigeria[J]. Journal of Food Biochemistry, 2019, 43: e12797. DOI:10.1111/jfbc.12797 |
| [73] |
GUTIÉRREZ-QUEQUEZANA L, VUORINEN A L, KALLIO H, YANG B R. Impact of cultivar, growth temperature and developmental stage on phenolic compounds and ascorbic acid in purple and yellow potato tubers[J]. Food Chemistry, 2020, 326: 126966. DOI:10.1016/j.foodchem.2020.126966 |
| [74] |
PIÑEROS-NIÑO C, NARVÁEZ-CUENCA C E, KUSHALAPPA A C, MOSQUERA T. Hydroxycinnamic acids in cooked potato tubers from Solanum tuberosum group Phureja[J]. Food Science & Nutriton, 2017, 5(3): 380-389. DOI:10.1002/fsn3.403 |
| [75] |
SHIMOMURA M, YOSHIDA H, FUJIUCHI N, ARIIZUMI T, EZURA H, FUKUDA N. Continuous blue lighting and elevated carbon dioxide concentration rapidly increase chlorogenic acid content in young lettuce plants[J]. Scientia Horticulturae, 2020, 272: 109550. DOI:10.1016/j.scienta.2020.109550 |
| [76] |
DJERRAB D, BERTRAND B, BREITLER J-C, LÉRAN S, DECHAMP E, CAMPA C, BARRACHINA C, CONEJERO G, ETIENNE H, SULPICE R. Photoperiod-dependent transcriptional modifications in key metabolic pathways in Coffea arabica[J]. Tree Physiology, 2020, 41: 302-316. DOI:10.1093/treephys/tpaa130 |
| [77] |
HALLMANN E. The influence of organic and conventional cultivation systems on the nutritional value and content of bioactive compounds in selected tomato types[J]. Journal of the Science of Food and Agriculture, 2012, 92: 2840-2848. DOI:10.1002/jsfa.5617 |
| [78] |
LUTHRIA D, SINGH A P, WILSON T, VORSA N, BANUELOS G S, VINYARD B T. Influence of conventional and organic agricultural practices on the phenolic content in eggplant pulp: plant-to-plant variation[J]. Food Chemistry, 2010, 121: 406-411. DOI:10.1016/j.foodchem.2009.12.055 |
| [79] |
HALLMANN E, REMBIA ŁKOWSK A E. Characterisation of antioxidant compounds in sweet bell pepper (Capsicum annuum L.) under organic and conventional growing systems[J]. Journal of the Science of Food and Agriculture, 2012, 92: 2409-2415. DOI:10.1002/jsfa.5624 |
| [80] |
CAMACHO-CRISTÓBAL J J, LUNAR L, LAFONT F, BAUMERT A, GONZÁLEZ-FONTES A. Boron deficiency causes accumulation of chlorogenic acid and caffeoyl polyamine conjugates in tobacco leaves[J]. Journal of Plant Physiology, 2004, 161: 879-881. DOI:10.1016/j.jplph.2003.12.003 |
| [81] |
FLORES I R, VÁSQUEZ-MURRIETA M S, FRANCO-HERNÁNDEZ M O, MÁRQUEZ-HERRERA C E, PONCE-MENDOZA A, LOPEZ-CORTÉZ M D S. Bioactive compounds in tomato (Solanum lycopersicum) variety saladette and their relationship with soil mineral content[J]. Food Chemistry, 2021, 344: 128608. DOI:10.1016/j.foodchem.2020.128608 |
| [82] |
KURT D, KINAY A. Effects of irrigation, nitrogen forms and topping on sun cured tobacco[J]. Industrial Crops & Products, 2021, 162: 113276. DOI:10.1016/j.indcrop.2021.113276 |
(责任编辑 马春敏)
 2023, Vol. 50
2023, Vol. 50