文章信息
基金项目
- 国家自然科学基金(42090041, 42030502);广西自然科学基金(2018GXNSFAA281328)
作者简介
- 许勇前(1997—),男,在读硕士生,研究方向为海洋微生物生态和纯培养,E-mail:Yongqian_Xu@outlook.com.
通讯作者
- 梁甲元(1986—),男,博士,副教授,研究方向为海洋微生物,E-mail:jyliang@gxu.edu.cn.
文章历史
- 收稿日期:2023-05-05
2. 广西南海珊瑚礁研究重点实验室,广西 南宁 530004;
3. 广西大学珊瑚礁研究中心,广西 南宁 530004
2. Guangxi Laboratory on the Study of Coral Reefs in the South China Sea, Nanning 530004, China;
3. Coral Reef Research Center of China, Nanning 530004, China
研究意义珊瑚礁生态系统是海洋四大生态系统之一,分布于热带和亚热带寡营养浅水区,极具生物和功能多样性,具有重要的生态价值和经济价值[1-2]。然而,在过去几十年里,活珊瑚覆盖率大幅度下降,全球珊瑚礁持续退化[3]。据Loya等[4]、Hoegh-Guldberg等[5]预测,到21世纪中叶,全球活珊瑚覆盖率会继续下降70%~90%。“珊瑚-虫黄藻”共生体是珊瑚礁生态系统的基础[6],虫黄藻为珊瑚提供了超过95%的光合能量,促进珊瑚钙化和生长[7];同时,珊瑚宿主也会为虫黄藻提供二氧化碳、氮、磷以及其他无机物,以维持虫黄藻的光合作用[8]。然而,受全球气候变化和人类活动干扰,珊瑚与虫黄藻的共生关系易破裂,进而导致珊瑚白化、死亡[9]。因此,了解“珊瑚-虫黄藻”共生体的环境适应能力具有重要意义。前人研究进展珊瑚的环境适应能力与其共生虫黄藻的群落结构有关。一般而言,在一定范围内,虫黄藻密度与珊瑚的耐受性存在正相关。基于我国南海珊瑚礁区大空间尺度的调查表明,具有较高虫黄藻密度的珊瑚宿主一般也表现出较强的抗逆性[10]。室内模拟实验也证实,霜鹿角珊瑚(Acropora pruinosa)的热耐受性与共生虫黄藻密度呈正相关[11]。此外,虫黄藻的群落组成也影响珊瑚的环境耐受性。例如,主导系群分别为Cladocopium和Durusdinium的Pocillopora珊瑚,两者的热耐受性显著不同。[12] Berkelmans等[13]将多孔鹿角珊瑚(Acropora millepora)移植到温度较高的海域后,占据主导优势的C2亚系群逐渐被Durusdinium属所取代,且珊瑚的耐热性增加了1~1.5七。因此,了解虫黄藻的群落结构对于评估珊瑚的环境适应能力十分必要。本研究切入点“珊瑚-虫黄藻”共生体对环境的响应是一个动态变化的过程,多种环境因素均会引起虫黄藻群落结构的变化,而目前的研究多聚焦于空间尺度上。例如,Chen等[14]基于两个气候带73个调查站位的研究发现,南海珊瑚共生虫黄藻群落组成整体呈现出由高纬度的Cladocopium主导转变为中、低纬度Cladocopium + Durusdinium主导的空间分布模式。而在时间尺度上,Gong等比较了霜鹿角珊瑚(Acroporapruinosa)和鹿角杯型珊瑚(Pocillopora damicornis)共生虫黄藻密度的昼夜变化情况,发现白天的虫黄藻密度显著高于晚上。基于昼夜节律变化的监控数据已对虫黄藻响应短时间尺度的变化特征有了初步的认识,而跨越季节和偶发性极端气候的生态调查则可以更好的解析虫黄藻对环境变化的响应特征。因此,亟待补充长时间尺度,特别是季节性的生态调查。拟解决的关键问题本研究以涠洲岛一种代表性的热敏感枝状霜鹿角珊瑚为研究对象,分别在2019—2020年连续4个季节定点采集珊瑚样品,同时收集了采样期间的遥感卫星环境数据,通过分析4个季节的虫黄藻密度、群落组成、群落结构与环境变量的相关性来揭示季节性环境变化驱动虫黄藻的生态效应。
1 材料与方法 1.1 样品标记与采集珊瑚样品于2019年7、10月以及2020年1、4月在涠洲岛珊瑚礁区固定苗圃(21。8.27' N,109。12.6' E)采集。每次采集5个霜鹿角珊瑚枝(长约5 cm), 采样深度为6~7 m, 共计20个样品。珊瑚出水后用无菌海水冲洗3遍,避免外源虫黄藻的干扰[16],随后立即装入无菌密封袋并置于液氮速冻,回到实验室进行后续实验操作。
1.2 涠洲岛珊瑚礁区环境数据通过Giovanni Ocean Color tool(https://giovanni.gsfc.nasa.gov/giovanni)获取涠洲岛珊瑚礁区从2019年7月至2020年7月的遥感数据(MODIS-Aqua 4 km)。获取的遥感数据类别包括:表层海水温度(Sea surface temperature, SST, ℃)、光合有效辐射(Photosynthetically active radiation, PAR, E/m2 ・d)、海水叶绿素a浓度(Chlorophyll a, mg/m3)、490 nm下的入射光漫射衰减系数(Kd490, Kd/m)和颗粒有机碳(Particulate organic carbon, POC,mg/m3)。
1.3 虫黄藻密度和叶绿素a测定用无菌海水高压冲洗霜鹿角珊瑚样品,直至珊瑚组织全部脱落,确保将虫黄藻全部冲洗下来。收集全部冲洗液,测量并记录体积,吸取1 mL冲洗液,8 000 r/min离心10 min后去上清,加入4% 甲醛1 mL进行固定。虫黄藻计数参考朱昔恩等[17]的方法。剪取锡箔纸包裹珊瑚骨骼表面,称量锡箔纸重量,以5 cm × 5 cm的锡箔纸称量作为标准, 根据锡箔纸重量与表面积的相关性计算珊瑚表面积[18]。单位面积的虫黄藻密度根据Xu等[19]的方法进行计算。
取适量珊瑚组织(约100 g)浸泡于90%丙酮中,在4 ℃黑暗条件下萃取24 h。然后在酶标仪内分别读取波长664、647、630 nm处的吸光值,并参考赵永平等[20]的方法计算叶绿素a浓度。
1.4 DNA提取、高通量测序和数据分析每枝珊瑚剪取3份组织用于生物学重复,每份组织90 mg, 包含碳酸钙骨骼、组织和黏液成分, 用于基因组DNA的提取,4个季节20个珊瑚枝共计60份样品。DNA提取参照海洋动物基因组DNA提取试剂盒(TIANGEN, DP324)说明书操作。以质检合格的DNA为模板,使用特异性引物ITSintfor2(5' -GATTGCAGAACTCCGTG-3')[21]和ITS2-reverse(5 ' -GGGATCCATATGCTTAAGT TCAGCGGGT-3' [22]对虫黄藻的ITS2区域进行扩增。PCR反应体系、反应条件和高通量测序流程参考Liang等[23]的方法。测序完成后,对二代测序下机数据进行严格的数据拆分和质控拼接,通过DADA2和Deblur对优化后的数据进行降噪处理,去除高通量测序数据中可能存在的错误,获得ASV代表序列和丰度表。将ASV丰度表与Chen等[14]建立的虫黄藻数据库比对,获得亚系群的代表序列及丰度表,并用于后续虫黄藻群落统计分析。原始数据保存于NCBI的SRA数据库(Accession number:PRJNA880748)。
1.5 统计分析和绘图对4个季节的虫黄藻密度和叶绿素a浓度进行单因素方差分析,采用Tukey' s HSD多均值比较进行事后检验,显著性水平设为0.05,数据均以平均值±标准差表示。基于虫黄藻数据库比对后的丰度表,通过冗余分析(Redundancy analysis, RDA)计算4个季节虫黄藻群落结构之间的统计差异,检验环境参数和虫黄藻群落结构之间的相关性。群落组成所展示的数据为3个生物学重复合并为1个样本。RDA分析在软件R(version 4.2.1) 中通过vegan和ggplot2完成。其他绘图在origin2023中完成,统计分析在SPSS27.0中进行。
2 结果与分析 2.1 涠洲岛珊瑚礁区环境参数的季节变化涠洲岛珊瑚礁区环境参数的季节波动显著,几乎所有环境参数的极值均出现在夏季或冬季(图 1)。其中,SST和PAR的变化趋势较为类似,最低值均出现在冬季,最高值分别出现在夏季和春末,且两者随季节交替平稳变化,表现出较强的共线性;而Chlorophyll a、Kd490和POC的极值也出现夏季或冬季,但这3种环境参数的最高值均出现在冬季,最低值均在夏季,且这3种环境参数与季节变化并未表现出较强的关联性。整体来看,在采样时间范围内,涠洲岛气候表现出夏季高温强光和冬季低温弱光的环境特征。
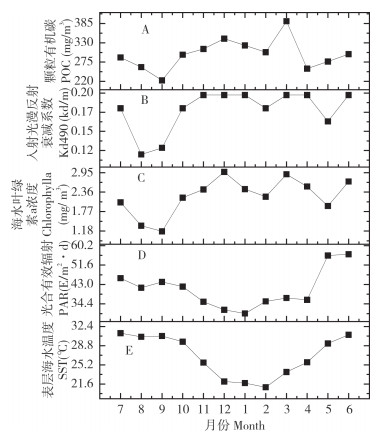
|
| 图 1 涠洲岛珊瑚礁区2019—2020年各月环境参数 Fig. 1 Environmental parameters of Weizhou Island coral reef area in each month from 2019 to 2020 |
2.2 涠洲岛珊瑚礁区虫黄藻密度的季节变化
由图 2可知,不同季节珊瑚共生虫黄藻的密度在0.56X 106〜2.33 X 106 cells/cm2之间,夏、秋、冬、春的平均虫黄藻密度分别为1.04(±0.33)x106、1.17(±0.37)x106、1.58(±0.49)x106、1.14(±0.27)x106 cells/cm2,以冬季虫黄藻密度最高、夏季最低,冬季虫黄藻密度约为夏季的1.5倍。对4个季节的虫黄藻密度进行单因素方差分析,冬季虫黄藻密度显著高于夏季(F=4.431,P<0.01)。总体而言,虫黄藻密度表现出夏季低冬季高的特点。
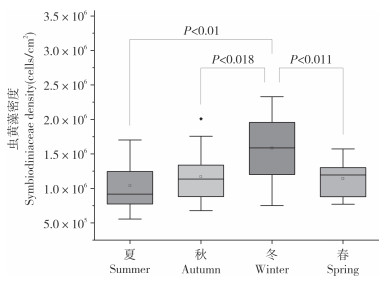
|
| 图 2 霜鹿角珊瑚虫黄藻密度的季节变化 Fig. 2 Seasonal variation of density of Symbiodiniaceae in Acropora pruinose |
2.3 霜鹿角珊瑚叶绿素a浓度的季节变化
由图 3可知,不同季节霜鹿角珊瑚叶绿素a的浓度为9.53〜13.67 yg/cm2。夏、秋、冬、春的平均叶绿素a浓度分别为10.55(±0.81)、11.17(±0.75)、13.05(±0.48)、11.36(±0.91)yg / cm2。其中,叶绿素a浓度最高,其次为春季和秋季,夏季最低。冬季叶绿素a浓度约为夏季的1.24倍。对4个季节的叶绿素a浓度进行单因素方差分析,冬季显著高于其它3个季节(F=10.02, P<0.05)。总体而言,叶绿素a浓度变化趋势类似于虫黄藻密度,同样表现出夏季低冬季高的特点。
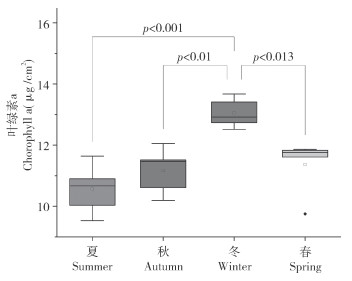
|
| 图 3 霜鹿角珊瑚叶绿素a浓度的季节变化 Fig. 3 Seasonal variation of concentration of chlorophyll a in Acropora pruinose |
2.4 涠洲岛珊瑚礁区虫黄藻群落组成的季节性变化
虫黄藻多样性分析结果(图 4)显示,共检测到117种ASVs且均为Cladocopium属。亚系群水平上,4个季节的虫黄藻组成较为相似,C1亚系群的相对丰度介于79.35%~82.97%之间,在4个季节的霜鹿角珊瑚中占据绝对主导优势,其相对丰度无明显变化;C1ca、C1p、C72和Cspc等亚系群的相对丰度分别介于3.10%~6.19%、2.81%~3.95%、2.15%~2.93%、1.62%~2.64%之间,但其相对丰度随季节交替而显著变化(F=2.107, P<0.01)。
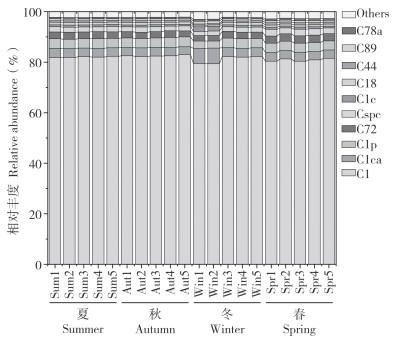
|
| 图 4 霜鹿角珊瑚虫黄藻群落组成的季节变化 Fig. 4 Seasonal variation of composition of Symbiodiniaceae communities in Acropora pruinose |
2.5 涠洲岛珊瑚礁区环境因子与虫黄藻群落结构的相关性
RDA分析(图 5)显示,SST、PAR、Kd490、Chlorophyll a和POC均与虫黄藻群落结构变化存在关联性(Permutation test, P<0.05), 其中RDA1、RDA2分别解释虫黄藻群落结构变化的54.97% 和18.50%。整体来看,春季和秋季虫黄藻群落结构相似度较高,而夏季和冬季的差异较大。此外,夏季虫黄藻群落结构与SST和PAR呈正相关,与Kd490、Chlorophyll a、POC则呈负相关,且环境参数对于冬季虫黄藻群落结构的影响与夏季相反, PAR、SST、Kd490、Chlorophyll a、POC对于虫黄藻群落结构的影响依次减弱,SST和PAR对虫黄藻群落结构的影响高于Kd490、Chlorophyll a、POC。
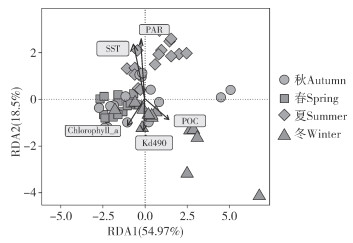
|
| 图 5 虫黄藻群落结构与季节环境参数之间的关联性分析 Fig. 5 Correlation analysis between community structure of Symbiodiniaceae and seasonal environmental parameters |
3 讨论 3.1 虫黄藻密度和叶绿素a浓度的季节变化模式
虫黄藻密度和叶绿素a是珊瑚的重要生理参数,常用于评估珊瑚的环境适应能力[24]。本研究分析了涠洲岛珊瑚礁区4个季节的霜鹿角珊瑚共生虫黄藻密度和叶绿素a浓度变化情况,两者均呈现出夏季低冬季高的规律。这与其他野外调查的结果一致[25-32,但影响虫黄藻密度和叶绿素a浓度的因素较多,如表层海水温度、光合有效辐射、营养盐浓度、洋流等[19]。这两种生理参数的变化情况不能用一种或几种变量来解释,需要综合考虑研究区域的总体季节变化。
涠洲岛地处南海北部的高纬度地区,与其他海域相比,表层海水温度和光合有效辐射是影响珊瑚生长的主要因素[33],同时也可能是驱动虫黄藻密度和叶绿素a浓度季节变化的重要因素。有研究通过控制温度和光照等变量,探究珊瑚共生虫黄藻的密度变化情况。Nielsen等[34]对多孔鹿角珊瑚(Acropora millepora) 进行了为期10周的温度胁迫,证实23.0 ℃低温胁迫后虫黄藻密度显著上升,而高温29.5 ℃胁迫后虫黄藻密度显著降低。此外,体外培养的虫黄藻在高温和强光胁迫下,密度也会降低[35]。上述研究发现,高温和强光会降低虫黄藻密度,同时涠洲岛夏季高温强光和冬季低温弱光的季节特征可能是造导致虫黄藻密度和叶绿素a浓度变化的重要因素。
高温和强光会破坏虫黄藻的叶绿体光合系统Ⅱ, 导致活性氧过量合成[36]。活性氧是光合作用的正常产物,但浓度过高时会泄漏到珊瑚组织中,破坏珊瑚细胞和DNA, 进而导致虫黄藻细胞死亡或被排出珊瑚体外[37-38]。而在温度较低的冬季,珊瑚为了提高对光的捕获效率,虫黄藻由单层排列转变为多层排列,虫黄藻密度和叶绿素a浓度间接增加[39]。因此,表层海水温度和光合有效辐射的季节性波动可能通过影响虫黄藻的光合作用,进而驱动其密度变化。
3.2 珊瑚共生虫黄藻主导系群的季节响应特征本研究发现,C1亚系群虫黄藻与霜鹿角珊瑚稳定共生,且始终占据主导地位。一般情况下,成体珊瑚与多种虫黄藻共生,且只有一种虫黄藻系群能占据主导地位[6, 40-42]。中国南海低纬度珊瑚礁区[43]、中国香港[44-45]、日本冲绳[46]、韩国济州岛[47]以及大堡礁南部[48]等亚热带低纬度地区的珊瑚几乎均由C1亚系群主导,而其他类型的虫黄藻相对丰度较低,这与本研究在涠洲岛的调查结果一致。C1亚系群虫黄藻与霜鹿角珊瑚高度稳定的共生关系可能是长期自然选择和演化的结果。珊瑚受到外界环境胁迫时,其共生虫黄藻可通过重组[12, 49]或者替换[50-51]两种机制来适应环境。但也有研究表明,新的共生关系并不稳定,当外界环境胁迫消除后,虫黄藻会恢复为原始群落组成[52-56]。因此,长期野外调查发现,珊瑚共生虫黄藻的主导系群基本不变[57]。这进一步表明,尽管环境变化会短暂影响珊瑚与虫黄藻的共生关系,但在长时间尺度上,珊瑚依旧会与最适应环境的虫黄藻形成共生关系。
C1亚系群虫黄藻对高温敏感⑷],广泛分布于印度-太平洋和大西洋-加勒比海域[58]。在受低温胁迫的珊瑚礁区,C1亚系群占据了主导地位[46-47]。而C15、C3u、D1.a等耐热型虫黄藻则更常见于SST较高和频繁发生热白化的热带低纬度礁区[59]。耐热型虫黄藻提高了珊瑚在高温环境中的存活率[53],却也减少了向珊瑚宿主转移的有机碳,降低了珊瑚宿主的钙化率和骨骼生长速率[60-62],甚至会使珊瑚更容易感染疾病[63]。在采样期间,涠洲岛的最高SST约为31。, 尚未达到C1亚系群的温度上限,耐热型虫黄藻并没有竞争优势[43]。另一方面,C1亚系群的温度适应范围较广,能够适应低温与富营养化环境,光合效率较高[64],而C1亚系群在低温下的强光合速率可保证珊瑚在冬季正常生长和碳酸钙骨骼形成[44]。因此,表层海水温度可能是驱动C1亚系群虫黄藻与霜鹿角珊瑚稳定共生的主要原因。
3.3 季节变化对虫黄藻群落结构的影响本研究中,涠洲岛珊瑚礁区霜鹿角珊瑚共生虫黄藻不同季节的群落结构主要受SST和PAR影响。已有研究表明,表层海水温度和光合有效辐射是影响虫黄藻群落结构的关键因素[43]。室内模拟实验也证实,杯型珊瑚(Pocillopora sp.)在受到温度胁迫的时候,虫黄藻群落结构随温度升高而发生转变,主导系群C42的相对丰度由76% 下降至25%[65]。此外,光合有效辐射强度也会影响珊瑚共生虫黄藻群落结构的转变。例如,红海萼形柱珊瑚(Stylophora pistillata)和加勒比海的山地星珊瑚(Orbicella faveolata)的主导类型虫黄藻随光合有效辐射减弱由Symbiodinium转变为Cladocopium [66-67]。可能受限于采样频率,本研究未发现主导系群发生替换的情况,C1亚系群始终占据主导地位,不随季节交替而显著变化,但次主导系群变化显著。
虫黄藻群落由相对丰度高于5%的主导系群和低于5 %且高度多样化的背景系群组成[68-69],两种类型的虫黄藻共同决定整体群落对环境变化的响应[70]。低丰度的背景虫黄藻比主导系群更活跃,在环境胁迫下取代主导系群后,“珊瑚- 虫黄藻”共生体的抗干扰能力增加,有助于珊瑚在极端环境下生存[71]。例如,在2005年东加勒比海域的珊瑚白化事件中,虫黄藻D1.a由背景系群转变为主导系群,提高了白化珊瑚的生存率,增强了珊瑚抵御热白化的能力[54]。因此,在涠洲岛表层海水温度和光合有效辐射季节性波动的环境下,背景系群虫黄藻相对丰度的显著上升可能有助于霜鹿角珊瑚生存。
4 结论本研究通过对涠洲岛霜鹿角珊瑚共生虫黄藻进行调查和分析,揭示了其密度变化、叶绿素a浓度、群落组成、群落结构的季节变化规律。结果表明,夏季高温强光致使虫黄藻密度和叶绿素a浓度显著降低,光合作用受损;而在冬季低温弱光下,为提高光捕获率,虫黄藻密度和叶绿素a浓度显著增加。并且,C1亚系群始终为4个季节的主导系群,可能与其高光合效率、较宽的温度耐受范围以及富氮环境耐受等生理特性有关。此外,表层海水温度和光合有效辐射通过影响虫黄藻背景系群丰度变化,进而导致群落结构差异显著。整体来看,季节性的表层海水温度和光合有效辐射波动是影响虫黄藻群落结构变化的主要因素。本研究以涠洲岛霜鹿角珊瑚为例,解析了低纬度礁区珊瑚共生虫黄藻群落的季节变化特征,有助于进一步揭示珊瑚微生物组的时空分布模式。
广西大学海洋学院王广华和俞小鹏老师在论文修改和样品采集等方面提供了帮助,谨此致谢
| [1] |
余克服. 珊瑚礁科学概论[M]. 北京: 科学出版社, 2018. YU K F. Introduction to the science of coral reefs[M]. Beijing: Science Press, 2018. |
| [2] |
吴家法, 李洁, 张偲. 鹿回头岸礁区4种造礁珊瑚中可培养细菌的多样性[J]. 广东农业科学, 2015, 42(2): 146-151. DOI:10.16768/j.issn.1004-874X.2015.02.003 WU J F, LI J, ZHANG S. Diversity of culturable bacteria associated with four scleractinian corals located in Luhuitou fringing reef[J]. Guangdong Agricultural Sciences, 2015, 42(2): 146-151. DOI:10.16768/j.issn.1004-874X.2015.02.003 |
| [3] |
HUGHES T P, BARNES M L, BELLWOOD D R, CINNER J E, CUMMING G S, JACKSON J B, KLEYPAS J, VAN DE LEEMPUT I A, LOUGH J M, MORRISON T H. Coral reefs in the Anthropocene[J]. Nature, 2017, 546(7656): 82-90. DOI:10.1038/nature22901 |
| [4] |
LOYA Y, SAKAI K, YAMAZATO K, NAKANO Y, SAMBALI H, VAN WOESIK R. Coral bleaching: The winners and the losers[J]. Ecology Letters, 2001, 4(2): 122-131. DOI:10.1046/j.1461-0248.2001.00203.x |
| [5] |
HOEGH-GULDBERG O, KENNEDY E V, BEYER H L, MCCLENNEN C, POSSINGHAM H P. Securing a long-term future for coral reefs[J]. Trends in Ecology & Evolution, 2018, 33(12): 936-944. DOI:10.1016/j.tree.2018.09.006 |
| [6] |
BAKER A C. Flexibility and specificity in coral-algal symbiosis: Diversity, ecology, and biogeography of Symbiodinium[J]. Annual Review of Ecology Evolution and Systematics, 2003, 34: 661-689. DOI:10.1146/annurev.ecolsys.34.011802.132417 |
| [7] |
FALKOWSKI P G, DUBINSKY Z, MUSCATINE L, PORTER J W. Light and the bioenergetics of a symbiotic coral[J]. BioScience, 1984, 34(11): 705-709. DOI:10.2307/1309663 |
| [8] |
MUSCATINE L, PORTER J W. Reef corals: Mutualistic symbioses adapted to nutrient-poor environments[J]. Bioscience., 1977, 27(7): 454-460. DOI:10.2307/1297526 |
| [9] |
KNOWLTON N. The future of coral reefs[J]. Proceedings of the National Academy of Sciences, 2001, 98(10): 5419-5425. DOI:10.1073/pnas.091092998 |
| [10] |
QIN Z, YU K, WANG Y, XU L, HUANG X, CHEN B, LI Y, WANG W, PAN Z. Spatial and intergeneric variation in physiological indicators of corals in the South China Sea: Insights into their current state and their adaptability to environmental stress[J]. Journal of Geophysical Research: Oceans, 2019, 124(5): 3317-3332. DOI:10.1029/2018JC014648 |
| [11] |
YU X, YU K, HUANG W, LIANG J, QIN Z, CHEN B, YAO Q, LIAO Z. Thermal acclimation increases heat tolerance of the scleractinian coral Acropora pruinosa[J]. Science of The Total Environment, 2020, 733: 139319. DOI:10.1016/j.scitotenv.2020.139319 |
| [12] |
ROWAN R. Thermal adaptation in reef coral symbionts[J]. Nature, 2004, 430(7001): 742-742. DOI:10.1038/430742a |
| [13] |
BERKELMANS R, VAN OPPEN M J H. The role of zooxanthellae in the thermal tolerance of corals: A "nugget of hope" for coral reefs in an era of climate change[J]. Proceedings of the Royal Society B-Biological Sciences, 2006, 273(1599): 2305-2312. DOI:10.1098/rspb.2006.3567 |
| [14] |
CHEN B, YU K, LIANG J, HUANG W, WANG G, SU H, QIN Z, HUANG X, PAN Z, LUO W, LUO Y, WANG Y. Latitudinal variation in the molecular diversity and community composition of Symbiodiniaceae in coral from the South China Sea[J]. Frontiers in Microbiology, 2019, 10: 1278. DOI:10.3389/fmicb.2019.01278 |
| [15] |
GONG S, LI G, LIANG J, XU L, TAN Y, JIN X, XIA X, YU K. Day-night cycle as a key environmental factor affecting coral-Symbiodiniaceae symbiosis[J]. Ecological Indicators, 2023, 146: 109890. DOI:10.1016/j.ecolind.2023.109890 |
| [16] |
FUJISE L, SUGGETT D J, STAT M, KAHLKE T, BUNCE M, GARDNER S G, GOYEN S, WOODCOCK S, RALPH P J, SEYMOUR J R, SIBONI N, NITSCHKE M R. Unlocking the phylogenetic diversity, primary habitats, and abundances of free-living Symbiodiniaceae on a coral reef[J]. Molecular Ecology, 2021, 30(1): 343-360. DOI:10.1111/mec.15719 |
| [17] |
朱昔恩, 林原有, 项桂德, 熊建华, 陈田聪, 陈晓汉, 张彬. 盐度、温度和光照对广西沿海牟氏角毛藻生长的影响[J]. 广东农业科学, 2022, 49(1): 136-141. DOI:10.16768/j.issn.1004-874X.2022.01.016 ZHU X, LIN E, XIANG G, XIONG J, CHEN S, CHEN X, ZHANG L. Effects of salinity, temperature and light on the growth of chaetoceros muelleri from the coast of Guangxi[J]. Guangdong Agricultural Sciences, 2022, 49(1): 136-141. DOI:10.16768/j.issn.1004-874X.2022.01.016 |
| [18] |
李淑, 余克服, 陈天然, 施祺, 陈特固. 珊瑚共生虫黄藻密度的季节变化及其与珊瑚白化的关系一一以大亚湾石珊瑚为例[J]. 热带海洋学报, 2011, 30(2): 39-45. DOI:10.11978/j.issn.1009-5470.2011.02.039 LI S, YU K F, CHEN T R, SHI Q, CHEN T G. Seasonal patterns of densities of symbiotic zooxanthellae in scleractinian corals from Daya Bay, northern South China Sea, and relation to coral bleaching[J]. Journal of Tropical Oceanography, 2011, 30(2): 39-45. DOI:10.11978/j.issn.1009-5470.2011.02.039 |
| [19] |
XU L, YU K, LI S, LIU G, TAO S, SHI Q, CHEN T, ZHANG H. Interseasonal and interspecies diversities of Symbiodinium density and effective photochemical efficiency in five dominant reef coral species from Luhuitou fringing reef, northern South China Sea[J]. Coral Reefs, 2017, 36(2): 477-487. DOI:10.1007/s00338-016-1532-y |
| [20] |
赵永平, 惠亚云, 朱亚, 赵盟, 张毅, 付献欧, 马雪莉. 施氮对干旱胁迫紫苏叶绿素含量和光合作用的影响[J]. 广东农业科学, 2019, 46(10): 63-68. DOI:10.16768/j.issn.1004-874X.2019.10.010 ZHAO Y, HUI Y, ZHU Y, ZHAO M, ZHANG Y, FU X, MA X. Effects of nitrogen application on chlorophyll content and photosynthesis of Perilla frutescens under drought stress[J]. Guangdong Agricultural Sciences, 2019, 46(10): 63-68. DOI:10.16768/j.issn.1004-874X.2019.10.010 |
| [21] |
LAJ EUNESSE T, TRENCH R. Biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal sea anemone Anthopleura elegantissima (Brandt)[J]. The Biological Bulletin, 2000, 199(2): 126-134. DOI:10.2307/1542872 |
| [22] |
COLEMAN A W, SUAREZ A, GOFF L J. Molecular delineation of species and syngens in volvocacean green algae (chlorophyta)1[J]. Journal of Phycology, 1994, 30(1): 80-90. DOI:10.1111/j.0022-3646.1994.00080.x |
| [23] |
LIANG J, DENG C, YU K, GE R, XU Y, QIN Z, CHEN B, WANG Y, SU H, HUANG X, HUANG W, WANG G, GONG S. Cross-linked regulation of coral-associated dinoflagellates and bacteria in Pocillopora sp. during high-temperature stress and recovery[J]. Microorganisms, 2021, 9(9): 1972. DOI:10.3390/microorganisms9091972 |
| [24] |
HINRICHS S, PATTEN N L, WAITE A M. Temporal variations in metabolic and autotrophic indices for Acropora digitifera and Acropora spicifera-implications for monitoring projects[J]. PLOS ONE, 2013, 8(5): e63693. DOI:10.1371/journal.pone.0063693 |
| [25] |
SHU L I, KEFU Y U, TIANRAN C, QI S H I, TEGU C. Seasonal patterns of densities of symbiotic zooxanthellae in scleractinian corals from Daya Bay, northern South China Sea, and relation to coral bleaching[J]. Journal of Tropical Oceanography, 2011, 30(2): 39-45. DOI:10.11978/j.issn.1009-5470.2011.02.039 |
| [26] |
STIMSON J. The annual cycle of density of zooxanthellae in the tissues of field and laboratory-held Pocillopora damicornis (Linnaeus)[J]. Journal of Experimental Marine Biology and Ecology, 1997, 214(1-2): 35-48. |
| [27] |
FAGOONEE I., WILSON H. B., HASSELL M. P., TURNER J. R.. The dynamics of Zooxanthellae populations: A long-term study in the field[J]. Science, 1999, 283(5403): 843-845. DOI:10.1126/science.283.5403.843 |
| [28] |
FITT W K, MCFARLAND F K, WARNER M E, CHILCOAT G C. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching[J]. Limnology and Oceanography, 2000, 45(3): 677-685. DOI:10.4319/lo.2000.45.3.0677 |
| [29] |
ULSTRUP K E, HILL R, VAN OPPEN M J H, LARKUM A W D, RALPH P J. Seasonal variation in the photo-physiology of homogeneous and heterogeneous Symbiodinium consortia in two scleractinian corals[J]. Marine Ecology Progress Series, 2008, 361: 139-150. DOI:10.3354/meps07360 |
| [30] |
SAWALL Y, AL-SOFYANI A, BANGUERA-HINESTROZA E, VOOLSTRA C R. Spatio-temporal analyses of Symbiodinium physiology of the coral Pocillopora verrucosa along large-scale nutrient and temperature gradients in the Red Sea[J]. Plos One, 2014, 9(8): e103179. DOI:10.1371/journal.pone.0103179 |
| [31] |
JANDANG S, VIYAKARN V, YOSHIOKA Y, SHINZATO C, CHAVANICH S. The seasonal investigation of Symbiodiniaceae in broadcast spawning, Acropora humilis and brooding, Pocillopora cf. damicornis corals[J]. PeerJ, 2022, 10: e13114. DOI:10.7717/peerj.13114 |
| [32] |
HAIYANG Z, MEIXIA Z, YU Z, LI L, GUOHUI L, HONGQIANG Y, HONGQIANG Y. Seasonal monitoring of photosynthesis characteristics of scleractinian corals in the northern South China Sea[J]. Marine Geology Letters, 2021, 37(6): 84-91. |
| [33] |
MWAURA J, GRIMSDITCH G, KILONZO J, AMIYO N, OBURA D. Zooxanthellae densities are highest in summer months in equatorial corals in Kenya[J]. Western Indian Ocean Journal of Marine Science, 2009, 8(2): 980. DOI:10.4314/wiojms.v8i2.56980 |
| [34] |
NIELSEN J J V, KENKEL C D, BOURNE D G, DESPRINGHERE L, MOCELLIN V J L, BAY L K. Physiological effects of heat and cold exposure in the common reef coral Acropora millepora[J]. Coral Reefs, 2020, 39(2): 259-269. DOI:10.1007/s00338-019-01881-x |
| [35] |
LESSER M P. Phylogenetic signature of light and thermal stress for the endosymbiotic dinoflagellates of corals (Family Symbiodiniaceae)[J]. Limnology and Oceanography, 2019, 64(5): 1852-1863. DOI:10.1002/lno.11155 |
| [36] |
WARNER M E, FITT W K, SCHMIDT G W. Damage to photosystem Ⅱ in symbiotic dinoflagellates: A determinant of coral bleaching[J]. Proceedings of the National Academy of Sciences, 1999, 96(14): 8007-8012. DOI:10.1073/pnas.96.14.8007 |
| [37] |
SZABO M, LARKUM A W D, VASS I. A review: The role of reactive oxygen species in mass coral bleaching[M]. Cham: Springer International Publishing, 2020: 459-488. DOI:10.1007/978-3-030-33397-3_17
|
| [38] |
LESSER M P. Oxidative stress causes coral bleaching during exposure to elevated temperatures[J]. Coral Reefs, 1997, 16(3): 187-192. DOI:10.1007/s003380050073 |
| [39] |
VENN A, LORAM J, DOUGLAS A. Photosynthetic symbioses in animals[J]. Journal of Experimental Botany, 2008, 59(5): 1069-1080. DOI:10.1093/jxb/erm328 |
| [40] |
ROWAN R, KNOWLTON N, BAKER A, JARA J. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching[J]. Nature, 1997, 388(6639): 265-269. DOI:10.1038/40843 |
| [41] |
LAJEUNESSE T C. Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs[J]. Marine Biology, 2002, 141(2): 387-400. DOI:10.1007/s00227-002-0829-2 |
| [42] |
LITTLE ANGELA F., VAN OPPEN MADELEINE J. H., WILLIS BETTE L.. Flexibility in algal endosymbioses shapes growth in reef corals[J]. Science, 2004, 304(5676): 1492-1494. DOI:10.1126/science.1095733 |
| [43] |
CHEN B, YU K, LIANG J, HUANG W, WANG G, SU H, QIN Z, HUANG X, PAN Z, LUO W, LUO Y, WANG Y. Latitudinal variation in the molecular diversity and community composition of Symbiodiniaceae in coral from the South China Sea[J]. Frontiers in Microbiology, 2019, 10: 1278. DOI:10.3389/fmicb.2019.01278 |
| [44] |
NG T Y, ANG P. Low symbiont diversity as a potential adaptive strategy in a marginal non-reefal environment: a case study of corals in Hong Kong[J]. Coral Reefs, 2016, 35(3): 941-957. DOI:10.1007/s00338-016-1458-4 |
| [45] |
WONG J C Y, THOMPSON P, XIE J Y, QIU J W, BAKER D M. Symbiodinium clade C generality among common scleractinian corals in subtropical Hong Kong[J]. Regional Studies in Marine Science, 2016, 8: 439-444. DOI:10.1016/j.rsma.2016.02.005 |
| [46] |
REIMER J D, TAKISHITA K, MARUYAMA T. Molecular identification of symbiotic dinoflagellates (Symbiodinium spp.) from Palythoa spp.(Anthozoa: Hexacorallia) in Japan[J]. Coral Reefs, 2006, 25(4): 521-527. DOI:10.1007/s00338-006-0151-4 |
| [47] |
DE PALMAS S, DENIS V, RIBAS-DEULOFEU L, LOUBEYRES M, WOO S, HWANG S, SONG J, CHEN C. Symbiodinium spp. associated with high-latitude scleractinian corals from Jeju Island, South Korea[J]. Coral Reefs, 2015, 34(3): 919-925. DOI:10.1007/s00338-015-1286-y |
| [48] |
HOEGH-GULDBERG O, FINE M. Low temperatures cause coral bleaching[J]. Coral Reefs, 2004, 23(3): 444. DOI:10.1007/s00338-004-0401-2 |
| [49] |
BAKER A C, STARGER C J, MCCLANAHAN T R, GLYNN P W. Corals' adaptive response to climate change[J]. Nature, 2004, 430(7001): 741. DOI:10.1038/430741a |
| [50] |
BUDDEMEIER R, FAUTIN D. Coral bleaching as an adaptive mechanism[J]. Bioscience, 1993, 43(43). DOI:10.2307/1312064 |
| [51] |
FAUTIN D G, BUDDEMEIER R W. Adaptive bleaching: A general phenomenon[J]. Hydrobiologia, 2004, 530: 459-467. DOI:10.1007/s10750-004-2642-z |
| [52] |
THORNHILL D J, LAJEUNESSE T C, KEMP D W, FITT W K, SCHMIDT G W. Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion[J]. Marine Biology, 2006, 148(4): 711-722. DOI:10.1007/s00227-005-0114-2 |
| [53] |
JONES A M, BERKELMANS R, VAN OPPEN M J H, MIEOG J C, SINCLAIR W. A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization[J]. Proceedings of the Royal Society B: Biological Sciences, 2008, 275(1641): 1359-1365. DOI:10.1098/rspb.2008.0069 |
| [54] |
LAJEUNESSE T C, SMITH R T, FINNEY J, OXENFORD H. Outbreak and persistence of opportunistic symbiotic dinoflagellates during the 2005 Caribbean mass coral 'bleaching' event[J]. Proceedings of the Royal Society B: Biological Sciences, 2009, 276(1676): 4139-4148. DOI:10.1098/rspb.2009.1405 |
| [55] |
SAMPAYO E M, RIDGWAY T, BONGAERTS P, HOEGH-GULDBERG O. Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type[J]. Proceedings of the National Academy of Sciences, 2008, 105(30): 10444-10449. DOI:10.1073/pnas.0708049105 |
| [56] |
COFFROTH M A, POLAND D M, PETROU E L, BRAZEAU D A, HOLMBERG J C. Environmental symbiont acquisition may not be the solution to warming seas for reef-building corals[J]. PLoS One, 2010, 5(10): e13258. DOI:10.1371/journal.pone.0013258 |
| [57] |
GOULET T L. Most corals may not change their symbionts[J]. Marine Ecology Progress Series, 2006, 321: 1-7. DOI:10.3354/meps321001 |
| [58] |
LAJEUNESSE T C. "Species" radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the miocene-pliocene transition[J]. Molecular Biology and Evolution, 2005, 22(3): 570-581. DOI:10.1093/molbev/msi042 |
| [59] |
LAJEUNESSE T C, PETTAY D T, SAMPAYO E M, PHONGSUWAN N, BROWN B, OBURA D O, HOEGH-GULDBERG O, FITT W K. Long-standing environmental conditions, geographic isolation and host-symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium[J]. Journal of Biogeography, 2010, 37(5): 785-800. DOI:10.1111/j.1365-2699.2010.02273.x |
| [60] |
STAT M, GATES R D. Clade D Symbiodinium in scleractinian corals: A "nugget" of hope, a selfish opportunist, an ominous sign, or all of the above?[J]. Journal of Marine Biology, 2011, 2011. DOI:10.1155/2011/730715 |
| [61] |
JONES A, BERKELMANS R. Potential costs of acclimatization to a warmer climate: Growth of a reef coral with heat tolerant vs. sensitive symbiont types[J]. PloS One, 2010, 5(5): e10437. DOI:10.1371/journal.pone.0010437 |
| [62] |
CUNNING R, GILLETTE P, CAPO T, GALVEZ K, BAKER A C. Growth tradeoffs associated with thermotolerant symbionts in the coral Pocillopora damicornis are lost in warmer oceans[J]. Coral Reefs, 2015, 34(1): 155-160. DOI:10.1007/s00338-014-1216-4 |
| [63] |
SHORE-MAGGIO A, CALLAHAN S M, AEBY G S. Trade-offs in disease and bleaching susceptibility among two color morphs of the Hawaiian reef coral, Montipora capitata[J]. Coral Reefs, 2018, 37(2): 507-517. DOI:10.1007/s00338-018-1675-0 |
| [64] |
BAKER D M, ANDRAS J P, JORDAN-GARZA A G, FOGEL M L. Nitrate competition in a coral symbiosis varies with temperature among Symbiodinium clades[J]. The ISME Journal, 2013, 7(6): 1248-1251. DOI:10.1038/ismej.2013.12 |
| [65] |
ZHU W, LIU X, ZHU M, LI X, YIN H, HUANG J, WANG A, LI X. Responses of Symbiodiniaceae shuffling and microbial community assembly in thermally stressed Acropora hyacinthus[J]. Frontiers in Microbiology, 2022, 13: 832081. DOI:10.3389/fmicb.2022.832081 |
| [66] |
EZZAT L, FINE M, MAGUER J F, GROVER R, FERRIER-PAGES C. Carbon and nitrogen acquisition in shallow and deep holobionts of the scleractinian coral S. pistillata[J]. Frontiers in Marine Science, 2017, 4(102). DOI:10.3389/fmars.2017.00102 |
| [67] |
BAKER D M, FREEMAN C J, WONG J C Y, FOGEL M L, KNOWLTON N. Climate change promotes parasitism in a coral symbiosis[J]. The ISME Journal, 2018, 12(3): 921-930. DOI:10.1038/s41396-018-0046-8 |
| [68] |
BOULOTTE N M, DALTON S J, CARROLL A G, HARRISON P L, PUTNAM H M, PEPLOW L M, VAN OPPEN M J. Exploring the Symbiodinium rare biosphere provides evidence for symbiont switching in reef-building corals[J]. The ISME Journal, 2016, 10(11): 2693-2701. |
| [69] |
QUIGLEY K M, DAVIES S W, KENKEL C D, WILLIS B L, MATZ M V, BAY L K. Deep-sequencing method for quantifying background abundances of Symbiodinium types: Exploring the rare Symbiodinium biosphere in reef-building corals[J]. PLoS One, 2014, 9(4): e94297. DOI:10.1371/journal.pone.0094297 |
| [70] |
FABINA N S, PUTNAM H M, FRANKLIN E C, STAT M, GATES R D. Symbiotic specificity, association patterns, and function determine community responses to global changes: Defining critical research areas for coral-Symbiodinium symbioses[J]. Global Change Biology, 2013, 19(11): 3306-3316. DOI:10.1111/gcb.12320 |
| [71] |
ZIEGLER M, EGUfLUZ V M, DUARTE C M, VOOLSTRA C R. Rare symbionts may contribute to the resilience of coral-algal assemblages[J]. The ISME Journal, 2018, 12(1): 161-172. DOI:10.1038/ismej.2017.151 |
(责任编辑 邹移光)
 2023, Vol. 50
2023, Vol. 50




