文章信息
基金项目
- 广东省农业科学院科技创新战略专项资金(高水平农科院建设)(XTXM202202,202122TD);广东省基础与应用基础研究基金(2021A1515012401,2021A1515010521)
作者简介
- 余志会(1996—),男,在读硕士生,研究方向为鸡球虫生化代谢,E-mail:1090023510@qq.com.
通讯作者
- 廖申权(1981—),女,博士,研究员,研究方向为鸡球虫生化代谢,E-mail: lsq6969@163.com.
文章历史
- 收稿日期:2023-06-03
2. 仲恺农业工程学院动物科技学院,广东 广州 510225;
3. 温氏食品集团股份有限公司,广东 云浮 527400
2. College of Animal Science and Technology, Zhongkai University of Agriculture and Engineering, Guangzhou 510225, China;
3. Wen's Foodstuffs Group Co., Ltd., Yunfu 527400, China
寄生原虫是一类单细胞真核生物,包括利什曼原虫(Leishmania spp.)、锥虫(Trypanosoma spp.)、疟原虫(Plasmodium spp.)、刚地弓形虫(Toxoplasma gondii)、隐孢子虫(Cryptosporidium spp.)和艾美耳球虫(Eimeria spp.)等,所引起的寄生原虫病严重危害人类与动物健康,并造成严重的经济损失[1-4]。嘌呤碱基是核苷酸合成的前体,在细胞分裂、生长、信号转导、能量代谢和维生素合成等多种生理过程中都具有重要作用。寄生原虫的入侵、发育和繁殖需要大量的嘌呤核苷酸,嘌呤核苷酸参与细胞和寄生原虫的多个生化过程,在生物体和寄生原虫中发挥重要作用,如核苷酸为核酸生物合成提供前体物质,ATP为通用能量载体,核苷酸衍生物为多个生物合成反应提供重要前体物质[5]。生物体内,细胞可以通过从头合成和补救途径两种不同方式合成嘌呤核苷酸,以从头合成途径为主;但寄生原虫只能利用补救途径合成嘌呤核苷酸,通过从宿主细胞摄取核酸分解产生的核苷和碱基在磷酸核糖转移酶(Phosphoribosyltransferase, PRT)作用下合成嘌呤核苷酸[6-8]。寄生原虫参与嘌呤核苷酸合成的主要为腺嘌呤磷酸核糖转移酶(Adenine phosphoribosyltransferase, APRT)和6-氧代嘌呤核糖转移酶(6-oxopurine phosphoribosy ltransferase),后者在大多数寄生原虫中以次黄嘌呤 - 鸟嘌呤 - 黄嘌呤磷酸核糖转移酶(Hypoxanthine-guanine-xanthine phosphoribosy ltransferase, HGXPRT)形式存在。腺嘌呤在APRT的催化作用下转化为嘌呤核苷酸;次黄嘌呤、鸟嘌呤和黄嘌呤在HGXPRT的催化作用下分别转化为次黄嘌呤核苷酸、鸟嘌呤核苷酸和黄嘌呤核苷酸,其中5-磷酸核糖焦磷酸(Phosphoribosylpyrophosphate, PRPP)是嘌呤补救途径的主要底物。寄生原虫的生长发育需要大量的嘌呤核苷酸,APRT和HGXPRT作为虫体嘌呤补救途径关键酶,在寄生原虫嘌呤补救生物合成及生长发育过程中起重要作用[9-12]。由于寄生原虫的嘌呤补救途径明显区别于宿主的从头合成途径,因而原虫嘌呤补救途径关键酶成为抗原虫药物靶标研究的热点之一,为抗寄生原虫药物研发提供了新思路。近年来,越来越多的研究关注寄生原虫嘌呤磷酸核糖转移酶的生物学特征与功能,并以其为药物靶点进行抑制剂筛选研究[8, 13]。本文就寄生原虫嘌呤磷酸核糖转移酶的基本特征、生物学功能及其抑制剂研发等研究进展作一综述,以期为抗原虫药靶发现与抗原虫药物研制提供新的思路与理论依据。
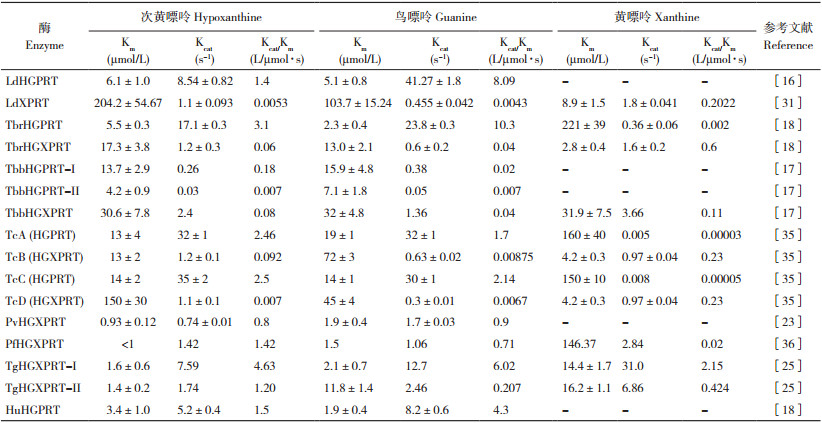
1 寄生原虫PRT的基本特征 1.1 寄生原虫HGXPRT的基本特征寄生原虫普遍存在HGXPRT。研究显示,杜氏利什曼原虫(L. donovani)的嘌呤磷酸核糖转移酶包括次黄嘌呤 - 鸟嘌呤磷酸核糖转移酶(Hypoxanthine-guanine phosphoribosyltransferase, LdHGPRT)和黄嘌呤磷酸核糖转移酶(Xanthine phosphoribosyltransferase, LdXPRT)两个成员,其中LdHGPRT由211个氨基酸残基组成,相对分子量为23.6 kD;LdXPRT由241个氨基酸残基组成,相对分子量为26 kD,两者氨基酸序列同源性达33%[14]。Zarella-boitz等[15]发现,LdHGPRT和LdXPRT的羧基端均存在由1个三肽分子组成的过氧化物酶体转导肽,两者均定位于利什曼原虫的糖酵解酶体,其中糖酵解酶体为动基体目寄生原虫所特有的微体,含有糖酵解反应的大多数酶。研究表明,LdHGPRT中保守的Ser-Tyr氨基酸残基是该蛋白家族的特征,对于嘌呤核苷酸的磷酸核糖化至关重要[16]。
布氏锥虫(T. brucei)嘌呤磷酸核糖转移酶由TbHGPRT-Ⅰ、TbHGPRT-Ⅱ和TbHGXPRT共3个同工酶组成。Dolezelova等[17]研究发现,TbHGPRT-I和TbHGPRT-II均由210个氨基酸组成,两者同源性高达98%;TbHGXPRT由234个氨基酸组成,与TbHGPRT-I和TbHGPRT-II的同源性仅为56%。随后研究发现TbHGPRT-I、TbHGPRT-II和TbHGXPRT均以同源二聚体形式发挥作用[18]。Vidhya等[19]发现TbHGPRT-II和TbHGXPRT定位于糖酵解酶体,而TbHGPRT-I定位于细胞质。对虫体不同时期表达量差异分析发现,TbHGPRT-I和TbHGXPRT在前循环型和血流型虫体中表达水平一致,而TbHGPRT-II仅在前循环型虫体中表达[18]。研究显示,克氏锥虫(T. cruzi)嘌呤磷酸核糖转移酶存在TcA、TcB、TcC和TcD等4种亚型,其中TcA与TcC均由221个氨基酸组成,属于TcHGPRTs;TcB与TcD均由231个氨基酸组成,属于TcHGXPRTs;TcHGPRTs和TcHGXPRTs氨基酸序列同源性达35%[20]。研究发现,TcHGPRTs主要在急性期的血流型虫体中表达,而TcHGXPRTs主要在急性期发展至脑炎期的虫体中表达,以维持虫体在脑组织中持续感染[21]。
恶性疟原虫(P. falciparum)次黄嘌呤 - 鸟嘌呤 - 黄嘌呤磷酸核糖转移酶(PfHGXPRT)由231个氨基酸组成,相对分子量为26.2 kD;间日疟原虫(P. vivax)次黄嘌呤 - 鸟嘌呤磷酸核糖转移酶(PvHGPRT)由233个氨基酸组成,相对分子量为27.8 kD;PfHGXPRT与PvHGPRT氨基酸序列的同源性达77%[22-23],两者在低离子浓度和高离子浓度下分别以同型四聚体和二聚体形式存在。研究发现疟原虫HGXPRT蛋白极不稳定,导致疟原虫HGXPRT的重组、表达、纯化和晶体培养均不易实现,目前已解析PfHGXPRT与底物次黄嘌呤的共结晶,以及PfHGXPRT与抑制剂ImmucilllnHP复合物的晶体结构,发现PfHGXPRT每个亚基由6个α螺旋与11个β折叠组成,其中46~149氨基酸处于蛋白质核心区[24]。
刚地弓形虫(T. gondii)6-氧代嘌呤核糖转移酶存在TgHGXPRT-Ⅰ和TgHGXPRT-Ⅱ两种亚型。研究发现,编码这两种同工酶的为同一个基因Tghgxprt,其完整的编码阅读框为840 bp。Tghgxprt基因转录、翻译后,通过不同的剪接方式形成两种不同的cDNA,从而形成TgHGXPRT-Ⅰ和TgHGXPRT-Ⅱ两种亚型。其中TgHGXPRT-Ⅰ由230个氨基酸组成,相对分子量为26.7 kD;TgHGXPRT-Ⅱ由279个氨基酸组成,与前者相比,仅在羧基端多49个氨基酸,相对分子量为31.8 kD[25]。Chaudhary等[26]发现TgHGXPRT-Ⅰ定位于虫体细胞质,TgHGXPRT-Ⅱ定位于内膜复合物,该复合物为顶复门寄生原虫细胞质膜内独特的膜细胞器。
1.2 寄生原虫APRT的基本特征杜氏利什曼原虫腺嘌呤磷酸核糖转移酶(LdAPRT)由237个氨基酸残基组成,相对分子量为26.2 kD[27],定位于虫体细胞质[15]。Phillips等[28]解析了LdAPRT与底物腺嘌呤、产物AMP,以及硫酸盐和柠檬酸盐离子等结合的晶体结构,发现LdAPRT属于Ⅰ型磷酸核糖转移酶,与该家族其他PRT的晶核结构相似,包含由13个氨基酸残基组成的PRPP核糖环结合的结构域,但在底物腺嘌呤结合的结构域存在差异;利什曼原虫APRT羧基端均存在延伸结构,以组成二聚体的接合面,且APRT活性位点由二聚体的两个亚基共同组成,推测利什曼原虫APRT二聚体结构对其催化活性至关重要。
布氏锥虫腺嘌呤磷酸核糖转移酶由TbAPRT1和TbAPRT2两个成员组成,在布氏锥虫基因组数据库中可注释到Tbaprt1和Tbaprt2两个基因,TbAPRT1和TbAPRT2氨基酸序列的同源性仅为22%,TbAPRT1和TbAPRT2与LdAPRT氨基酸序列的相似性分别为52% 和27%;序列比对分析发现,TbAPRT1属于Ⅰ型磷酸核糖转移酶,而TbAPRT2与乳清酸磷酸核糖转移酶(Orotate phosphoribosyltransferase, OPRT)具有结构相似的活性中心[29-30]。研究发现,TbAPRT1定位于细胞质,在血流型和前循环型锥虫中均有表达;TbAPRT2定位于糖酵解酶体,仅在前循环型锥虫中表达[29]。目前仍未发现疟原虫与弓形虫存在APRT,推测可能存在同工酶或代偿路径。
2 寄生原虫PRT的生物学功能 2.1 寄生原虫HGXPRT的生物学功能杜氏利什曼原虫利用LdHGPRT和LdXPRT两种酶催化不同的6- 氧代嘌呤底物,其中LdHGPRT催化底物次黄嘌呤和鸟嘌呤,而LdXPRT具有特殊的底物特异性,可催化黄嘌呤、次黄嘌呤和鸟嘌呤3种底物,黄嘌呤是LdXPRT的最适底物(表 1)[14]。Patel等[31]进一步分析LdXPRT底物特异性机制,发现LdXPRT(I209V)突变体对黄嘌呤的亲和力降低,突变后的催化功能类似HGXPRT,提示I209为LdXPRT底物特异性的关键位点。早期研究尝试构建杜氏利什曼原虫Δhgprt/Δxprt双缺失突变株,但均未成功,提示LdHGPRT和LdXPRT可能在利什曼原虫嘌呤核苷酸合成中起重要作用[14, 32]。随后Boitz等[33]构建了条件性杜氏利什曼原虫Δhgprt/Δxprt双缺失突变株,证实LdHGPRT和LdXPRT在虫体嘌呤核苷酸合成中均发挥关键作用。已有研究发现,黄嘌呤磷酸核糖转移酶(Xanthine phosphoribosyltransferase, XPRT)只存在利什曼原虫以及一些特定真菌和细菌中,哺乳动物缺乏XPRT[34],因而LdXPRT作为抗利什曼原虫药物靶点研究备受关注。
|
研究发现布氏锥虫嘌呤磷酸核糖转移酶存在TbHGPRT-Ⅰ、TbHGPRT-Ⅱ和TbHGXPRT亚型,每种亚型对6- 氧代嘌呤的亲和力存在差异,TbHGPRT-Ⅰ和TbHGPRT-Ⅱ可以利用次黄嘌呤和鸟嘌呤,对黄嘌呤无催化活性;TbHGXPRT可以催化次黄嘌呤、鸟嘌呤和黄嘌呤3种底物,且对3种底物的催化效率相近(表 1)[18]。Dolezelova等[17]利用RNAi技术将Tbhgprt-Ⅰ、Tbhgprt-Ⅱ和Tbhgxprt基因同时表达沉默,发现TbHGPRT-Ⅰ、TbHGPRT-Ⅱ和TbHGXPRT对感染期布氏锥虫的生存至关重要。克氏锥虫TcHGPRT包括TcA和TcC两个亚型,可以催化底物次黄嘌呤和鸟嘌呤,几乎不能利用黄嘌呤;TcHGXPRT包括TcB和TcD两个亚型,可以利用次黄嘌呤、鸟嘌呤和黄嘌呤3种底物,对黄嘌呤的亲和力高[35]。由于克氏锥虫嘌呤磷酸核糖转移酶存在4种亚型,因此以该酶为潜在药物靶标筛选出的抑制剂需同时抑制TcHGPRT和TcHGXPRT活性,这些结果可为后续抑制剂筛选研究提供基础。
恶性疟原虫PfHGXPRT可以利用次黄嘌呤、鸟嘌呤和黄嘌呤3种底物,其中鸟嘌呤为最适底物;而间日疟原虫PvHGPRT只能利用次黄嘌呤和鸟嘌呤,不能催化黄嘌呤合成嘌呤核苷酸[36],推测PfHGXPRT与PvHGPRT对黄嘌呤催化活性的差异可能与恶性疟原虫更易于从按蚊体内摄取高浓度黄嘌呤相关。研究表明,PfHGXPRT的酶活性需要低浓度PRPP与次黄嘌呤进行活化,其最佳活化条件为:PfHGXPRT浓度为37 μmol/L,PfHGXPRT与底物PRPP和次黄嘌呤的比值为1: 7.4:2.2,活化后的PfHGXPRT最大反应速率可达8.37 μmol/min·mg,是未活化酶的10.8倍,推测活化过程与PfHGXPRT聚合为具有更高活性的四聚体相关;然而高浓度的底物可以抑制PfHGXPRT酶活性,推测可能是高浓度底物PRPP或次黄嘌呤与PfHGXPRT紧密结合从而抑制产物的生成[37],这些结果提示最佳的PfHGXPRT酶活性反应体系是体外评价抑制剂效果时需考虑的重要因素。
刚地弓形虫6- 氧代嘌呤核糖转移酶的TgHGXPRT-Ⅰ和TgHGXPRT-Ⅱ两种亚型均以四聚体存在且均具有催化活性,TgHGXPRT-Ⅱ与底物亲和力较TgHGXPRT-Ⅰ弱,其中Ⅱ型对鸟嘌呤的Km值较Ⅰ型高6倍[25]。根据已有研究结果,仅在弓形虫中发现同一基因编码的两个亚型磷酸核糖转移酶,且两个亚型均能维持虫体的繁殖与发育,具体机制仍不清晰,但推测两种功能性亚型磷酸核糖转移酶与弓形虫广泛的寄生宿主特性相关,可以有助于弓形虫在不同宿主中利用摄取或转运的嘌呤进行嘌呤核苷酸合成[26]。
2.2 寄生原虫APRT的生物学功能杜氏利什曼原虫腺嘌呤磷酸核糖转移酶(LdAPRT)可利用腺嘌呤和PRPP,其对腺嘌呤和PRPP的Km值分别为2.3、25.1 μmol/L[27]。Boitz等[38]构建杜氏利什曼原虫Δaprt缺失突变株,发现杜氏利什曼原虫前鞭毛体Δaprt缺失株在腺嘌呤底物环境中不能存活,在腺苷环境中存活率降低50%;杜氏利什曼原虫无鞭毛体Δaprt缺失株可以在腺嘌呤环境中存活;Δaprt缺失株也可以感染巨噬细胞,提示虫体可以从宿主细胞摄取多种腺苷来源,利用其他补救途径进行嘌呤核苷酸合成。此外,利什曼原虫不仅利用APRT合成嘌呤核苷酸,还可以利用腺苷激酶(Adenosine kinase, AK)合成AMP,以及利用腺嘌呤氨基水解酶(Adenine aminohydrolase, AAH)生成次黄嘌呤,再经HGPRT催化生成IMP,进而通过腺苷琥珀酸合成酶、腺苷琥珀酸裂解酶生成AMP[39]。利用代谢流分析发现,利什曼原虫主要运用腺嘌呤转化为次黄嘌呤,再生成IMP,进而转化为AMP进行嘌呤核苷酸合成,因此推测利什曼原虫腺嘌呤氨基水解酶在嘌呤核苷酸合成中起重要作用[40]。
布氏锥虫APRT存在TbAPRT1和TbAPRT2两种同工酶:TbAPRT1具有催化活性,对腺嘌呤的Km值为3.7 μmol/L;而TbAPRT2对腺嘌呤的Km值为253 μmol/L,与TbAPRT1相比,被认为几乎无催化活性[30]。序列比对分析发现,TbAPRT1属于Ⅰ型磷酸核糖转移酶,而TbAPRT2与乳清酸磷酸核糖转移酶(Orotate phosphoribosyltransferase, OPRT)具有结构相似的活性中心,推测TbAPRT2作用的底物可能是乳清酸。研究提示TbAPRT1为布氏锥虫功能性的磷酸核糖转移酶。
弓形虫、疟原虫和隐孢子虫等不存在APRT酶催化活性,并且在基因组数据库中也未能注释到aprt基因[41]。研究发现弓形虫和隐孢子虫利用腺苷激酶催化腺苷ATP磷酸化生成AMP[42]。疟原虫缺失AK和APRT,利用腺苷脱氨酶(Ademosine deaminase, ADA)水解腺苷脱氨产生次黄苷,再通过嘌呤核苷磷酸化酶(Purine nucleoside phosphorylase, PNP)作用生成次黄嘌呤[43]。寄生原虫因其寄生的宿主环境各有不同,可能导致各原虫的嘌呤补救途径有所不同。
3 寄生原虫PRT的应用研究目前,以寄生原虫嘌呤磷酸核糖转移酶为潜在靶点的抑制剂研究较为广泛。研究结果显示,嘌呤基杂环化合物和核苷类似物等与嘌呤磷酸核糖转移酶的底物结构相似,因此在抗利什曼原虫、锥虫、疟原虫和弓形虫等的先导化合物研究中取得重要进展。
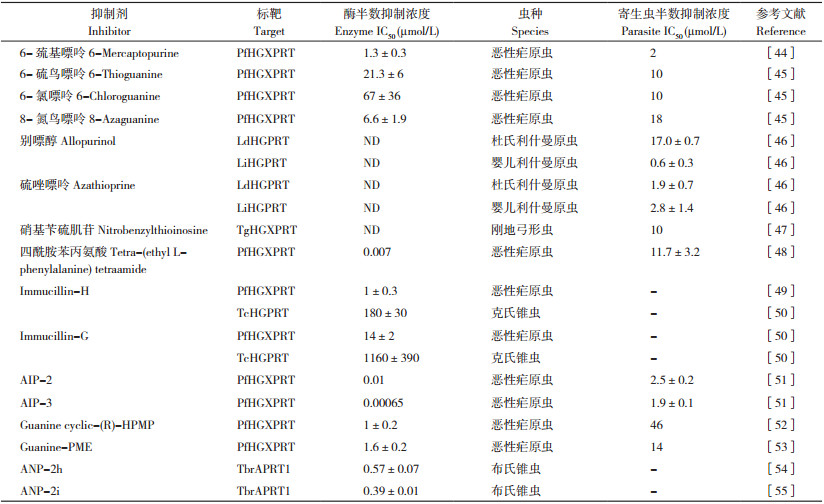
3.1 嘌呤基杂环化合物嘌呤基杂环化合物可抑制6- 氧代PRT活性,并且在体外能有效抑制寄生原虫繁殖。6- 巯基嘌呤、6- 硫鸟嘌呤、6- 氯嘌呤和8- 氮鸟嘌呤等能抑制PfHGXPRT酶活性,半数抑制浓度(IC50)为1.3~67 μmol/L;这些抑制剂也可以体外抑制恶性疟原虫的繁殖,IC50值为2~18 μmol/L[44-45]。研究发现,别嘌醇和硫唑嘌呤可以抑制体外利什曼原虫繁殖,对杜氏利什曼原虫的IC50值为1.9~17 μmol/L,对婴儿利什曼原虫的IC50值为0.6~2.8 μmol/L(表 2)[46]。已有研究发现嘌呤基杂环化合物中别嘌醇的抗原虫活性较好。
|
3.2 核苷类似物
核苷及核苷类似物是嘌呤磷酸核糖转移酶的天然底物,核苷类似物等抑制剂的设计成为抑制剂开发的有效策略之一。硝基苄硫肌苷是TgHGXPRT的竞争性抑制剂,其对TgHGXPRT的IC50值为10 μmol/L,对宿主细胞没有明显毒性,高剂量(100 μmol/L)对未感染的宿主细胞没有显著的毒性作用[47]。Keough等[48]发现,四酰胺苯丙氨酸对PfHGXPRT的IC50值为7 nmol/L,其抑制活性较嘌呤基杂环类似物强。Immucillin也是一类核苷类似物,Immucillin-H和Immucillin-G能抑制PfHGXPRT和TcHGPRT活性,其中对PfHGXPRT的抑制活性比TcHGPRT强[20, 49],但未见Immucillin对恶性疟原虫和克氏锥虫体外繁殖抑制效果的报道。Hazleton等[50]发现,非环化Immucillin类似物(Acyclic immucillin phosphonate, AIP)可以有效抑制6- 氧代PRT活性,其中衍生物AIP-2和AIP-3对PfHGXPRT的抑制活性Ki值分别为10、0.65 nmol/L,具有很强的抑制活性;对体外恶性疟原虫繁殖的IC50值分别为2.5、1.9 μmol/L。此外,非环化核苷类似物(acyclic nucleoside phosphonates, ANP)也具有抑制活性,利用分子对接等分析发现,ANP衍生物不仅对6- 氧代嘌呤磷酸核糖转移酶具有抑制活性,而且能抑制APRT活性,其中衍生物ANP-2h和ANP-2i对TbrAPRT1的抑制活性Ki值分别为0.57、0.39 μmol/L[51-53];2(磷酸乙氧基)乙鸟嘌呤、2(磷酸乙氧基)乙次黄嘌呤、Guanine cyclic-(R)-HPMP和Guanine-PME对PfHGXPRT的抑制活性Ki值分别为0.1、0.3、1.0、1.6 μmol/L(表 2)[54-57]。
3.3 其他抑制剂异乙酸酯和醋酸熊果酸可以抑制PfHGXPT活性,且抑制作用呈量效关系,两者均对PfHGXPT表现出较强的亲和力,解离常数分别为0.0833、2.8396 μmol/L,被认为是具有潜力的抗疟药物[55]。研究发现楝科植物提取物对利什曼原虫APRT具有抑制作用,其中青花菜根、叶甲醇提取物分别使APRT的比活性降低84.5% 和80.1%;红果菜果、枝、叶甲醇提取物分别使APRT的比活性降低78.7%、90.8% 和90.3%。在芸香科植物提取物中发现3个呋喃喹诺酮类生物碱,可使APRT的比活性降低90.7%[58]。楝科植物提取物和呋喃喹诺酮类生物碱均具有酶抑制作用,这些化合物的抗利什曼原虫效果仍有待进一步开展体内外试验验证[59]。
4 展望寄生原虫感染引起利什曼原虫病、锥虫病、疟原虫病、弓形虫病和鸡球虫病等,不仅对畜牧业造成严重的经济损失,而且严重威胁人类健康[60-62]。目前,寄生原虫病的防治仍主要依赖抗原虫药物,随着抗原虫药物的长期、广泛应用,寄生原虫对药物的抗药性日益严重,已有抗原虫药物的毒副作用等严重限制了药物的临床应用,因而筛选新的抗原虫药物靶标并研制新型抗原虫药物成为当务之急。嘌呤核苷酸具有重要的生物学功能,在寄生原虫的入侵、发育和繁殖等多个阶段需要大量的嘌呤核苷酸。宿主主要采用从头途径合成嘌呤核苷酸,然而,原虫缺乏嘌呤从头合成途径,完全依赖补救途径进行嘌呤核苷酸的生物合成,寄生原虫与宿主的嘌呤代谢机制存在的明显差异使得寄生原虫嘌呤补救途径关键酶次黄嘌呤 - 鸟嘌呤 - 黄嘌呤磷酸核糖转移酶和腺嘌呤磷酸核糖转移酶成为备受关注的抗原虫药物靶标。近年来,应用生物化学、药物化学、基因编辑技术等,揭示了利什曼原虫、锥虫、疟原虫和弓形虫等寄生原虫嘌呤代谢机制、嘌呤代谢关键酶特征与生物学功能,并在此基础上针对嘌呤代谢关键酶次黄嘌呤 - 鸟嘌呤 - 黄嘌呤磷酸核糖转移酶和腺嘌呤磷酸核糖转移酶筛选特异性抑制剂,通过体内外验证试验发现了一系列具有良好抗原虫活性的先导化合物。目前,计算机辅助药物设计在抗病毒、抗肿瘤药物的研发中取得重要进展,极大推进了扎那米韦、伊马替尼等药物的研发效率并成功上市[63]。随着计算机辅助药物设计方法逐步成熟,在药物研发中发挥越来越重要的作用,日益成为新药研究的核心技术之一。因而,在前期基于寄生原虫嘌呤磷酸核糖转移酶(APRT和HGXPRT)筛选出系列先导化合物的基础上,进一步解析寄生原虫嘌呤磷酸核糖转移酶的分子结构,明晰药靶与配体的作用机制,利用计算机辅助虚拟筛选技术进行高通量筛选特异、高效、安全的活性抑制剂,并开展体内外试验验证抑制剂的抗原虫活性,从而筛选出更高效安全的抗寄生原虫先导化合物,这将为基于嘌呤磷酸核糖转移酶为药物靶标的抗原虫新药研发提供新思路。
| [1] |
邓敏儿, 李娜, 郭亚琼, 冯耀宇, 肖立华. CRISPR/Cas9系统在寄生原虫基因编辑中的应用[J]. 畜牧兽医学报, 2023, 54(1): 69-79. DOI:10.11843/j.issn.0366-6964.2023.01.007 DENG M E, LI N, GUO Y Q, FENG Y Y, XIAO L H. Application of CRISPR/Cas9 system on gene editing of parasitic protozoa[J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(1): 69-79. DOI:10.11843/j.issn.0366-6964.2023.01.007 |
| [2] |
KOURBELI V, CHONTZOPOULOU E, MOSCHOVOU K, PAVLOS D, MAVROMOUSTAKOS T, PAPANASTASIOU I P. An overview on target-based drug design against kinetoplastid protozoan infections: human african trypanosomiasis, chagas disease and leishmaniases[J]. Molecules, 2021, 26(15): 4629. DOI:10.3390/molecules26154629 |
| [3] |
熊杰, 陈建平, 陈晓光, 陈瑛, 冯耀宇, 高凤, 高珊, 顾福康, 黄兵, 梁爱华, 龙红岸, 赖德华, 伦照荣, 缪炜, 倪兵, 邱子健, 邵晨, 汪建国, 文建凡, 徐奎栋, 余育和, 张龙现, 张西臣, 赵元莙, 宋微波. 进展中的原生动物学研究: 热点领域与新格局[J]. 中国科学: 生命科学, 2019, 49(10): 1301-1322. DOI:10.1360/SSV-2019-0127 XIONG J, CHEN J P, CHEN X G, CHEN Y, FENG Y Y, GAO F, GAO S, GU F K, HUANG B, LIANG A H, LONG H A, LAI D H, LUN Z R, MIAO W, NI B, QIU Z J, SHAO C, WANG J G, WEN J F, XU K D, YU Y H, ZHANG L X, ZHANG X C, ZHAO Y J, SONG W B. Progress of protozoological studies in China: Hot spots and new patterns[J]. Scientia Sinica Vitae, 2019, 49(10): 1301-1322. DOI:10.1360/SSV-2019-0127 |
| [4] |
RANGEL G W, LLINÁS M. Re-envisioning anti-apicomplexan parasite drug discovery approaches[J]. Frontiers in Cellular and Infection Microbiology, 2021, 11: 691121. DOI:10.3389/fcimb.2021.691121 |
| [5] |
YANG Y, TANG T, LI X, MICHEL T, LING L, HUANG Z, MULAKA M, WU Y, GAO H, WANG L, ZHOU J, MEUNIER B, KE H, JIANG L, RAO Y. Design, synthesis, and biological evaluation of multiple targeting antimalarials[J]. Acta Pharmaceutica Sinica B, 2021, 11(9): 2900-2913. DOI:10.1016/j.apsb.2021.05.008 |
| [6] |
OWOLOY E A, ENEJOH O A, AKANBI O M, BANKOL E O M. Molecular docking analysis of Plasmodium falcipar um dihydroorotate dehydrogenase towards the design of effective inhibitors[J]. Bioinformation, 2020, 16(9): 672-678. DOI:10.6026/97320630016672 |
| [7] |
BARNADAS-CARCELLER B, MARTINEZ-PEINADO N, GOMEZ L C, ROS-LUCAS A, GABALDON-FIGUEIRA J C, DIAZ-MOCHON J J, GASCON J, MOLINA I J, PINEDA DE LAS INFANTAS Y V M J, ALONSO-PADILLA J. Identification of compounds with activity against Trypanosoma cruzi within a collection of synthetic nucleoside analogs[J]. Frontiers in Cellular and Infection Microbiology, 2022, 12: 1067461. DOI:10.3389/fcimb.2022.1067461 |
| [8] |
PARIHAR P S, PRATAP J V. The L. donovani Hypoxanthineguanine phosphoribosyl transferase (HGPRT) oligomer is distinct from the human homolog[J]. Biochemical and Biophysical Research Communications, 2020, 532(4): 499-504. DOI:10.1016/j.bbrc.2020.08.052 |
| [9] |
CASSERA M B, ZHANG Y, HAZLETON K Z, SCHRAMM V L. Purine and pyrimidine pathways as targets in Plasmodium falciparum[J]. Current Topics in Medicinal Chemistry, 2011, 11(16): 2103-2115. DOI:10.2174/156802611796575948 |
| [10] |
山丹, 刘群. 顶复门原虫嘌呤代谢研究进展[J]. 中国兽医杂志, 2012, 48(7): 54-57. SHAN D, LIU Q. Research advances in purine phosphoribosy ltransferases of Apicomplexa[J]. Chinese Journal of Veterinary Medicine, 2012, 48(7): 54-57. |
| [11] |
王冰. 恶性疟原虫次黄嘌呤-鸟嘌呤-黄嘌呤磷酸核糖转移酶细胞免疫保护作用的研究[D]. 上海: 第二军医大学, 2006. WANG B. Evaluation of cell-mediated protective immunity to hypoxanthine guanine xanthine phosphoribosyl transferase (HGXPRT) of Plasmodium falciparum[D]. Shanghai: The Second Military Medical University, 2006. |
| [12] |
余莉. 利用RNA干扰技术双通道抑制弓形虫嘌呤补救合成途径[D]. 合肥: 安徽医科大学, 2008. YU L. Dual inhibition of purine salvage pathways using RNA interference technology in Toxoplasma gondii [D]. Hefei: Anhui Medical University, 2008. |
| [13] |
GRUBE C D, GILL C P, ROY H. Development of a continuous assay for high throughput screening to identify inhibitors of the purine salvage pathway in Plasmodium falciparum[J]. SLAS Discovery, 2022, 27(2): 114-120. DOI:10.1016/j.slasd.2021.12.002 |
| [14] |
JARDIM A, BERGESON S E, SHIH S, CARTER N, LUCAS R W, MERLIN G, MYLER P J, STUART K, ULLMAN B. Xanthine phosphoribosyltransferase from Leishmania donovani. Molecular cloning, biochemical characterization, and genetic analysis[J]. Journal of Biological Chemistry, 1999, 274(48): 34403-34410. DOI:10.1074/jbc.274.48.34403 |
| [15] |
ZARELLA-BOITZ J M, RAGER N, JARDIM A, ULLMAN B. Subcellular localization of adenine and xanthine phosphoribosyl transferases in Leishmania donovani[J]. Molecular and Biochemical Parasitology, 2004, 134(1): 43-51. DOI:10.1016/j.molbiopara.2003.08.016 |
| [16] |
JARDIM A, ULLMAN B. The conserved serine-tyrosine dipeptide in Leishmania donovani hypoxanthine-guanine phosphoribosy ltransferase is essential for catalytic activity[J]. Journal of Biological Chemistry, 1997, 272(14): 8967-8973. DOI:10.1074/jbc.272.14.8967 |
| [17] |
DOLEZELOVA E, TERAN D, GAHUR A O, KOTRBOVA Z, PROCHAZKOVA M, KEOUGH D, SPACEK P, HOCKOVA D, GUDDAT L, ZIKOVA A. Evaluation of the Trypanosoma brucei 6-oxopurine salvage pathway as a potential target for drug discovery[J]. PLOS Neglected Tropical Diseases, 2018, 12(2): e0006301. DOI:10.1371/journal.pntd.0006301 |
| [18] |
TERAN D, DOLEZELOVA E, KEOUGH D T, HOCKOVA D, ZIKOVA A, GUDDAT L W. Crystal structures of Trypanosoma brucei hypoxanthine-guanine-xanthine phosphoribosyltransferase in complex with IMP, GMP and XMP[J]. FEBS Journal, 2019, 286(23): 4721-4736. DOI:10.1111/febs.14987 |
| [19] |
VIDHYA V M, PONNURAJ K. Structure-based virtual screening and computational study towards identification of novel inhibitors of hypoxanthine-guanine phosphoribosyltransferase of Trypanosoma cruzi[J]. Journal of Cellular Biochemistry, 2021, 122(11): 1701-1714. DOI:10.1002/jcb.30122 |
| [20] |
GLOCKZIN K, KOSTOMIRIS D, MINNOW Y V, SUTHAGAR K, CLINCH K, GAI S, BUCKLER J N, SCHRAMM V L, TYLER P C, MEEK T D. Kinetic characterization and inhibition of Trypanosoma cruzi hypoxanthine-guanine phosphoribosyltransferases[J]. Biochemistr y, 2022, 61(19): 2088-2105. DOI:10.1021/acs.biochem.2c00312 |
| [21] |
ROJO G, PÈLISSIER F, SANDOVAL-RODRIGUEZ A, BACIGALUPO A, GARCÍA V, PINTO R, ORTIZ S, BOTTO-MAHAN C, CATTAN P E, SOLARI A. Organs infected with Trypanosoma cruzi and DTU identification in the naturally infected rodent Octodon degus[J]. Experimental Parasitology, 2020, 215: 107931. DOI:10.1016/j.exppara.2020.107931 |
| [22] |
SARKAR D, GHOSH I, DATTA S. Biochemical characterization of Plasmodium falciparum hypoxanthine-guanine-xanthine phosphorybosyltransferase: role of histidine residue in substrate selectivity[J]. Molecular and Biochemical Parasitology, 2004, 137(2): 267-276. DOI:10.1016/j.molbiopara.2004.05.014 |
| [23] |
KEOUGH D T, HOCKOVá D, KREČMEROVá M, ČESNEK M, HOLý A, NAESENS L, BRERETON I M, WINZOR D J, DE JERSEY J, GUDDAT L W. Plasmodium vivax hypoxanthine-guanine phosphoribosyltransferase: a target for anti-malarial chemotherapy[J]. Molecular and Biochemical Parasitology, 2010, 173(2): 165-169. DOI:10.1016/j.molbiopara.2010.05.018 |
| [24] |
MINNOW Y V T, SUTHAGAR K, CLINCH K, DUCATI R G, GHOSH A, BUCKLER J N, HARIJAN R K, CAHILL S M, TYLER P C, SCHRAMM V L. Inhibition and mechanism of Plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase[J]. ACS Chemical Biology, 2022, 17(12): 3407-3419. DOI:10.1021/acschembio.2c00546 |
| [25] |
WHITE E L, ROSS L J, DAVIS R L, ZYWNO-VAN GINKEL S, VASANTHAKUMAR G, BORHANI D W. The two Toxoplasma gondii hypoxanthine-guanine phosphoribosyltransferase isozymes form heterotetramers[J]. Journal of Biological Chemistry, 2000, 275(25): 19218-19223. DOI:10.1074/jbc.M908879199 |
| [26] |
CHAUDHARY K, DARLING J A, FOHL L M, SULLIVAN W J, DONALD R G, PFEFFERKORN E R, ULLMAN B, ROOS D S. Purine salvage pathways in the apicomplexan parasite Toxoplasma gondii[J]. Journal of Biological Chemistry, 2004, 279(30): 31221-31227. DOI:10.1074/jbc.M404232200 |
| [27] |
BASHOR C, DENU J M, BRENNAN R G, ULLMAN B. Kinetic mechanism of adenine phosphoribosyltransferase from Leishmania donovani[J]. Biochemistry, 2002, 41(12): 4020-4031. DOI:10.1021/bi0158730 |
| [28] |
PHILLIPS C L, ULLMAN B, BRENNAN RG, HILL C P. Crystal structures of adenine phosphoribosyltransferase from Leishmania donovani[J]. The BMEO Journal, 1999, 18(13): 3533-3545. DOI:10.1093/emboj/18.13.3533 |
| [29] |
LUSCHER A, LAMPREA-BURGUNDER E, GRAF F E, DE KONING H P, MASER P. Trypanosoma brucei adeninephosphoribosyltransferases mediate adenine salvage and aminopurinol susceptibility but not adenine toxicity[J]. International Journal for Parasitology: Drugs and Drug Resistance, 2014, 4(1): 55-63. DOI:10.1016/j.ijpddr.2013.12.001 |
| [30] |
GLOCKZIN K, MEEK T D, KATZFUSS A. Characterization of adenine phosphoribosyltransferase (APRT) activity in Trypanosoma brucei brucei: Only one of the two isoforms is kinetically active[J]. PLOS Neglected Tropical Diseases, 2022, 16(2): e0009926. DOI:10.1371/journal.pntd.0009926 |
| [31] |
PATEL B, PATEL D, PAPPACHAN A. Ile209 of Leishmania donovani xanthine phosphoribosyltransferase plays a key role in determining its purine base specificity[J]. FEBS Letters, 2021, 595(16): 2169-2182. DOI:10.1002/1873-3468.14162 |
| [32] |
HWANG H Y, ULLMAN B. Genetic analysis of purine metabolism in Leishmania donovani[J]. Journal of Biological Chemistry, 1997, 272(31): 19488-19496. DOI:10.1074/jbc.272.31.19488 |
| [33] |
BOITZ J M, ULLMAN B. A conditional mutant deficient in hypoxanthine-guanine phosphoribosyl-transferase and xanthine phosphoribosyltransferase validates the purine salvage pathway of Leishmania donovani[J]. Journal of Biological Chemistry, 2006, 281(23): 16084-16089. DOI:10.1074/jbc.M600188200 |
| [34] |
PATEL B, PATEL D, PARMAR K, CHAUHAN R, SINGH D D, PAPPACHAN A. L. donovani XPRT: Molecular characterization and evaluation of inhibitors[J]. Biochimica et Biophysica Acta: Proteins and Proteomics, 2018, 1866(3): 426-441. DOI:10.1016/j.bbapap.2017.12.002 |
| [35] |
GLOCKZIN K, MENEELY K M, HUGHES R, MAATOUK S W, PIÑA G E, SUTHAGAR K, CLINCH K, BUCKLER J N, LAMB A L, TYLER P C, MEEK T D, KATZFUSS A. Kinetic and structural characterization of Trypanosoma cruzi hypoxanthine-guaninexanthine phosphoribosyltransferases and repurposing of transitionstate analogue inhibitors[J]. Biochemistry, 2023, 62(14): 2182-2201. DOI:10.1021/acs.biochem.3c00116 |
| [36] |
SUJAY SUBBAYYA I N, SUKUMARAN S, SHIVASHANKAR K, BALARAM H. Unusual substrate specificity of a chimeric hypoxanthine-guanine phosphoribosyltransferase containing segments from the Plasmodium falciparum and human enzymes[J]. Biochemical and Biophysical Research Communications, 2000, 272(2): 596-602. DOI:10.1006/bbrc.2000.2816 |
| [37] |
ROY S, NAGAPPA L K, PRAHLADARAO V S, BALARAM H. Kinetic mechanism of Plasmodium falciparum hypoxanthineguanine-xanthine phosphoribosyltransferase[J]. Molecular and Biochemical Parasitology, 2015, 204(2): 111-120. DOI:10.1016/j.molbiopara.2016.02.006 |
| [38] |
BOITZ J M, ULLMAN B. Adenine and adenosine salvage in Leishmania donovani[J]. Molecular and Biochemical Parasitology, 2013, 190(2): 51-55. DOI:10.1016/j.molbiopara.2013.06.005 |
| [39] |
BOITZ J M, ULLMAN B, JARDIM A, CARTER N S. Purine salvage in Leishmania: complex or simple by design?[J]. Trends in Parasitology, 2012, 28(8): 345-352. DOI:10.1016/j.pt.2012.05.005 |
| [40] |
MARTIN J L, YATES P A, SOYSA R, ALFARO J F, YANG F, BURNUM-JOHNSON K E, PETYUK V A, WEITZ K K, CAMP D G, SMITH R D, WILMARTH P A, DAVID L L, RAMASAMY G, MYLER P J, CARTER N S. Metabolic reprogramming during purine stress in the protozoan pathogen Leishmania donovani[J]. PLOS Pathogens, 2014, 10(2): e1003938. DOI:10.1371/journal.ppat.1003938 |
| [41] |
FOX B A, BZIK D J. Biochemistry and metabolism of Toxoplasma gondii: Purine and pyrimidine acquisition in Toxoplasma gondii and other Apicomplexa//Toxoplasma gondii. The model apicomplexan—perspectives and methods[M]. Academic Press: Cambridge, MA, USA, 2020: 397-449. DOI: 10.1016/B978-0-12-815041-2.00009-8.
|
| [42] |
KOUNI M H E. Adenosine metabolism in Toxoplasma gondii potential targets for chemotherapy[J]. Current Pharmaceutical Design, 2007, 13(6): 581-597. DOI:10.2174/138161207780162836 |
| [43] |
JARUWAT A, RIANGRUNGROJ P, UBONPRASERT S, SAE-UENG U, KUAPRASERT B, YUTHAVONG Y, LEARTSAKULPANICH U, CHITNUMSUB P. Crystal structure of Plasmodium falciparum adenosine deaminase reveals a novel binding pocket for inosine[J]. Archives of Biochemistry and Biophysics, 2019, 667: 6-13. DOI:10.1016/j.abb.2019.04.002 |
| [44] |
KLEJCH T, KEOUGH D T, KING G, DOLEŽELOVÁ E, ČESNEK M, BUDĚŠÍNSKÝ M, ZÍKOVÁ A, JANEBA Z, GUDDAT L W, HOCKOVÁ D. Stereo-defined acyclic nucleoside phosphonates are selective and potent inhibitors of parasite 6-oxopurine phosphoribosyltransferases[J]. Journal of Medicinal Chemistry, 2022, 65(5): 4030-4057. DOI:10.1021/acs.jmedchem.1c01881 |
| [45] |
KEOUGH D T, SKINNER-ADAMS T, JONES M K, NG A-L, BRERETON I M, GUDDAT L W, DE JERSEY J. Lead compounds for antimalarial chemotherapy: purine base analogs discriminate between human and P. falciparum 6-oxopurine phosphoribosyltransferases[J]. Journal of Medicinal Chemistry, 2006, 49(25): 7479-7486. DOI:10.1021/jm061012j |
| [46] |
AZZOUZ S, LAWTON P. In vitro effects of purine and pyrimidine analogues on Leishmania donovani and Leishmania infantum promastigotes and intracellular amastigotes[J]. Acta Parasitologica, 2017, 62(3): 582-588. DOI:10.1515/ap-2017-0070 |
| [47] |
EL KOUNI M H, GUARCELLO V, AL SAFARJALANI O N, NAGUIB F N. Metabolism and selective toxicity of 6-nitrobenzylthioinosine in Toxoplasma gondii[J]. Antimicrobial Agents and Chemotherapy, 1999, 43(10): 2437-2443. DOI:10.1128/AAC.43.10.2437 |
| [48] |
KEOUGH D T, REJMAN D, POHL R, ZBORNIKOVA E, HOCKOVA D, CROLL T, EDSTEIN M D, BIRRELL G W, CHAVCHICH M, NAESENS L M J, PIERENS G K, BRERETON I M, GUDDAT L W. Desig n of Plasmodium viva x hypoxanthine-g uanine phosphoribosyltransferase inhibitors as potential antimalarial therapeutics[J]. ACS Chemical Biology, 2018, 13(1): 82-90. DOI:10.1021/acschembio.7b00916 |
| [49] |
EVANS G B, TYLER P C, Schramm V L. Immucillins in infectious diseases[J]. ACS Infectious Diseases, 2018, 4(2): 107-117. DOI:10.1021/acsinfecdis.7b00172 |
| [50] |
HAZLETON K Z, HO M C, CASSERA M B, CLINCH K, CRUMP D R, ROSARIO JR I, MERINO E F, ALMO S C, TYLER P C, SCHRAMM V L. Acyclic immucillin phosphonates: second-generation inhibitors of Plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase[J]. Chemistry & Biology, 2012, 19(6): 721-730. DOI:10.1016/j.chembiol.2012.04.012 |
| [51] |
KEOUGH D T, HOCKOVA D, HOLY A, NAESENS L M, SKINNERADAMS T S, JERSEY J D, GUDDAT L W. Inhibition of hypoxanthineguanine phosphoribosyltransferase by acyclic nucleoside phosphonates: a new class of antimalarial therapeutics[J]. Journal of Medicinal Chemistry, 2009, 52(14): 4391-4399. DOI:10.1021/jm900267n |
| [52] |
DOLEZELOVA E, KLEJCH T, SPACEK P, SLAPNICKOVA M, GUDDAT L, HOCKOVA D, ZIKOVA A. Acyclic nucleoside phosphonates with adenine nucleobase inhibit Trypanosoma brucei adenine phosphoribosyltransferase in vitro[J]. Scientific Reports, 2021, 11(1): 13317. DOI:10.1038/s41598-021-91747-6 |
| [53] |
KALCIC F, FRYDRYCH J, DOLEZELOVA E, SLAPNICKOVA M, PACHL P, SLAVETINSKA L P, DRACINSKY M, HOCKOVA D, ZIKOVA A, JANEBA Z. C1'-Branched acyclic nucleoside phosphonates mimicking adenosine monophosphate: Potent inhibitors of Trypanosoma brucei adenine phosphoribosyltransferase[J]. European Journal of Medicinal Chemistry, 2021, 225: 113798. DOI:10.1016/j.ejmech.2021.113798 |
| [54] |
KLEJCH T, KEOUGH D T, CHAVCHICH M, TRAVIS J, SKACEL J, POHL R, JANEBA Z, EDSTEIN M D, AVERY V M, GUDDAT L W, HOCKOVA D. Sulfide, sulfoxide and sulfone bridged acyclic nucleoside phosphonates as inhibitors of the Plasmodium falciparum and human 6-oxopurine phosphoribosyltransferases: Synthesis and evaluation[J]. European Journal of Medicinal Chemistry, 2019, 183: 111667. DOI:10.1016/j.ejmech.2019.111667 |
| [55] |
OPOKU F, GOVENDER P P, POOE O J, SIMELANE M B C. Evaluating iso-mukaadial acetate and ursolic acid acetate as Plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase inhibitors[J]. Biomolecules, 2019, 9(12): 861. DOI:10.3390/biom9120861 |
| [56] |
MOHAMED B S, NGUYEN M C, WEIN S, UTTARO J P, ROBERT X, VIOLOT S, BALLUT L, JUGNARAIN V, MATHÉ C, CERDAN R, AGHAJARI N, PEYROTTES S. Purine containing carbonucleoside phosphonate analogues as novel chemotype for Plasmodium falciparum inhibition[J]. European Journal of Medicinal Chemistry, 2023, 258: 115581. DOI:10.1016/j.ejmech.2023.115581 |
| [57] |
CHEVIET T, LEFEBVRE-TOURNIER I, WEIN S, PEYROTTES S. Plasmodium purine metabolism and its inhibition by nucleoside and nucleotide analogues[J]. Journal of Medicinal Chemistry, 2019, 62(18): 8365-8391. DOI:10.1021/acs.jmedchem.9b00182 |
| [58] |
AMBROZIN A R, LEITE A C, SILVA M, VIEIRA P C, FERNANDES J B, THIEMANN O H, DA SILVA M F, OLIVA G. Screening of Leishmania APRT enzyme inhibitors[J]. Pharmazie, 2005, 60(10): 781-784. |
| [59] |
Soni M, Pratap JV. Development of novel anti-Leishmanials: The case for structure-based approaches[J]. Pathogens, 2022, 11(8): 950. DOI:10.3390/pathogens11080950 |
| [60] |
李结, 路鹏云. 广东农垦规模化猪场寄生虫流行病学调查[J]. 广东农业科学, 2017, 44(5): 132-136. DOI:10.16768/j.issn.1004-874X.2017.05.021 LI J, LU P Y. Survey of parasites infection in pigs of intensive farms in Guangdong state farms[J]. Guangdong Agricultural Sciences, 2017, 44(5): 132-136. DOI:10.16768/j.issn.1004-874X.2017.05.021 |
| [61] |
李娟, 廖申权, 赵爽, 戚南山, 吕敏娜, 吴彩艳, 林栩慧, 蔡海明, 胡俊菁, 张健騑, 谢明权, 孙铭飞. 重要食源性寄生虫流行新特点及防控策略[J]. 广东农业科学, 2021, 48(3): 123-132. DOI:10.16768/j.issn.1004-874X.2021.03.015 LI J, LIAO S Q, ZHAO S, QI N S, LYU M N, WU C Y, LIN X H, CAI H M, HU J J, ZHANG J F, XIE M Q, SUN M F. New epidemic characteristics and control strategies of important food-borne parasites[J]. Guangdong Agricultural Sciences, 2021, 48(3): 123-132. DOI:10.16768/j.issn.1004-874X.2021.03.015 |
| [62] |
廖申权, 戚南山, 吕敏娜, 吴彩艳, 李娟, 蔡海明, 林栩慧, 胡俊菁, 于林增, 张健騑, 谢明权, 孙铭飞. 鸡球虫病流行病学、防治药物与疫苗研究进展[J]. 广东农业科学, 2020, 47(11): 171-181. DOI:10.16768/j.issn.1004-874X.2020.11.019 LIAO S Q, QI N S, LYU M N, WU C Y, LI J, CAI H M, LIN X H, HU J J, YU L Z, ZHANG J F, XIE M Q, SUN M F. Research progress in the epidemiology, anticoccidial drugs and vaccines of avian coccidiosis[J]. Guangdong Agricultural Sciences, 2020, 47(11): 171-181. DOI:10.16768/j.issn.1004-874X.2020.11.019 |
| [63] |
ECE A. Computer-aided drug design[J]. BMC Chemistry, 2023, 17: 26-28. DOI:10.1186/s13065-023-00939-w |
(责任编辑 崔建勋)
 2023, Vol. 50
2023, Vol. 50




