文章信息
基金项目
- 广东省自然科学基金(2023A1515012102);广东省教育厅青年创新项目(2022KQNCX078);韶关学院博士科研启动经费项目(202112)
作者简介
- 叶红,博士,讲师,韶关学院生物与农业学院专任教师。主要从事植物免疫机制、园艺植物品质形成机理、植物遗传学等研究。主持广东省自然科学基金面上项目1项、广东省教育厅青年创新人才项目1项,参与国家级、省级和韶关市级项目多项。以第一作者在《Frontiers in Plant Science》《Plant Physiology and Biochemistry》《广东农业科学》《分子植物育种》等国内外期刊上发表学术论文多篇。叶红(1988—),女,博士,讲师,研究方向为植物生理,E-mail:19881212hong@163.com; 王玉昆,博士,副教授,现任职于韶关学院广东省粤北食药资源利用与保护重点实验室。主要从事园艺植物品质形成机理、植物遗传学等研究。主持广东省教育厅特色创新项目1项、韶关市科技计划项目1项,参与国家级、省级和韶关市级项目多项。已在SCI剘刊发表学术论文20余篇,中文核心期刊发表论文数篇。现为中国植物学会会员、中国作物学会会员.
通讯作者
- 王玉昆(1985—),男,博士,副教授,研究方向为植物生理,E-mail:wangyu_kun1@163.com.
文章历史
- 收稿日期:2023-07-13
由于植物无法移动,因此生存环境的变化几乎对其各生长发育阶段均有影响。为适应环境变化,植物进化出复杂的信号系统来感知各种生物和非生物刺激,并将这种能力保存并延续到下一代[1]。转录因子(Transcription Factors, TF)是生物体各种信号通路的重要组成部分,除了能对胞内信号进行响应外,还介导了植物对生物和非生物刺激的应答过程。WRKY TF是植物中最大的转录调控家族之一,其成员已在多种植物基因组中被鉴定。如萝卜(Raphanus sativus)[2]、空心菜(Ipomoea aquatica)[3]和苹果(Malus domestica)[4]等园艺植物的基因组中分别含有126、82、127个WRKY基因家族成员。
典型的WRKY DNA结合域的特征是在蛋白序列的N端有1个“WRKYGQK”的氨基酸基序,在其C端含有非典型的锌指结构基序。随着WRKY成员在不同植物中被鉴定,发现WRKY蛋白的结构和功能具有明显的多样性,其基因和蛋白质数量、内含子数量及DNA结合序列在不同植物之间存在差异。依据WRKY蛋白的一级结构,该家族成员可聚类到7个亚组,即Ⅰ、Ⅱa、Ⅱb、Ⅱc、Ⅱd、Ⅱe和Ⅲ亚组。WRKY基因通过其两端的特异性结构域结合靶基因启动子中的W-box顺式元件,从而激活或抑制靶基因转录[5]。越来越多的研究表明,WRKY TF几乎参与了植物所有的生长发育调控过程,且单个WRKY TF可能参与多个信号调节通路[6]。相比模式植物拟南芥和其他主要粮食作物,WRKY基因家族成员及其生物学功能在园艺植物中的挖掘和揭示还不够深入,但也取得了一些进展,因此有必要对已取得的成果进行归纳总结。本文综述了目前园艺植物中WRKY基因家族的研究进展,以期为今后更加深入研究该家族成员功能提供参考。
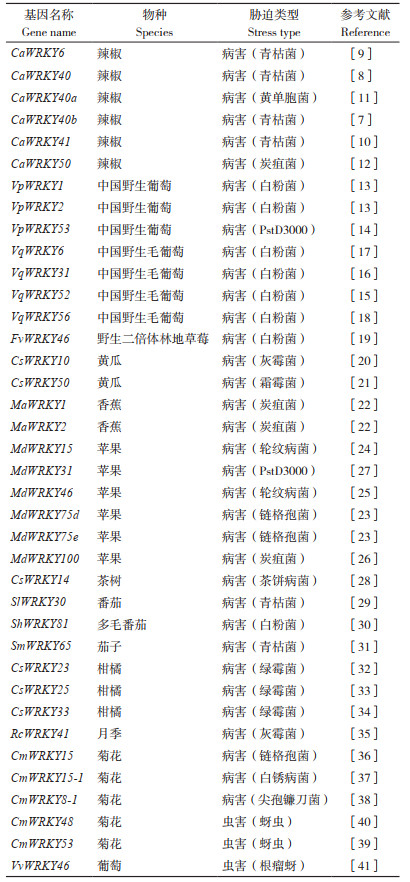
1 WRKY基因在园艺植物生物胁迫应答中的作用 1.1 WRKY基因在园艺植物病害中的作用植物在生长发育过程中会遭受一系列生物胁迫,而病原体攻击是其面临的一大威胁。在长期的进化过程中,植物获得了多种免疫应答能力,通过相应的免疫应答通路对病原体进行抵抗。WRKY TF相关研究结果(表 1)表明,该家族的许多成员在植物免疫应答相关的转录调控中发挥重要作用[5]。
|
在辣椒(Capsicum annuum)中,CaWRKY40b能够调控一系列免疫相关基因的表达对青枯菌(Ralstonia solanacearum)作出应答[7],过表达CaWRKY40基因能够调节超敏反应(Hypersensitive response, HR)相关基因和发病相关基因,从而增强辣椒对青枯菌的抗性[8]。CaWRKY6基因通过与CaWRKY40的启动子结合从而激活其表达,进而增强辣椒对青枯菌的抗性[9]。此外,CaWRKY41基因的表达受到青枯病菌的诱导,瞬时沉默该基因的表达使辣椒对青枯菌的感病性增强,说明该基因参与正向调控辣椒对青枯菌的抗性[10]。黄单胞菌外蛋白S(Xanthomonas outer protein S)能够与CaWRKY40a蛋白结合并增强其稳定性,从而降低辣椒对黄单胞菌的抗性[11]。CaWRKY50基因的表达受到炭疽菌(Colletotrichum musae)侵染的诱导,沉默该基因的表达增加了辣椒对炭疽菌的抗性,说明该基因是辣椒抗炭疽菌的负调控因子[12]。在中国野生葡萄(Vitis pseudoreticulata)中克隆到的VpWRKY1和VpWRKY2基因在其异位表达的拟南芥中增强了其对白粉病菌的抗性[13]。此外,白粉病菌侵染能上调VpWRKY53基因的表达,在拟南芥中异源过表达VpWRKY53基因增加了转基因拟南芥对番茄丁香假单胞菌PstDC3000的抗性[14]。从中国野生毛葡萄(Chinese wild V. quinquangularis)中克隆到的VqWRKY52基因也与白粉病菌的抗性有关,其在受到白粉病菌侵染24 h后显著表达。在拟南芥中异位表达VqWRKY52基因增强了转基因拟南芥对白粉病菌的抗性[15]。VqWRKY31基因也能对白粉病菌侵染作出响应,并促进代谢途径中基因的表达和许多抗病相关代谢产物的积累,包括二苯乙烯、类黄酮和原花青素,从而增强葡萄的白粉病抗性[16]。中国野生毛葡萄VqWRKY6能够和转录因子VqbZIP1互作形成转录复合体。共同过表达VqWRKY6和VqbZIP1后,叶片表面白粉菌菌丝扩繁速率显著慢于单独过表达VqWRKY6和单独过表达VqbZIP1的叶片,说明VqWRKY6和VqbZIP1协同作用能抑制白粉菌的生长,从而提高葡萄对白粉病的抗性[17]。VqWRKY56基因的表达受到白粉菌侵染的显著诱导,其在葡萄中的过表达降低了对白粉病的易感性[18]。另外,在拟南芥中过表达野生二倍体林地草莓((Fragaria vesca)WRKY46基因增强了转基因拟南芥对白粉病菌的抗性[19]。在黄瓜(Cucumis sativus)中,CsWRKY10在黄瓜对灰霉菌(Botrytis cinerea)的抗性中起关键作用。CsWRKY10的过表达显著增加了黄瓜对灰霉菌的敏感性。病原菌侵染后,转基因黄瓜植株过氧化氢酶、超氧化物歧化酶和过氧化物酶活性受到影响,导致活性氧(Reactive oxygen species, ROS) 含量降低。同时,CsWRKY10的过表达促进了灰霉菌的孢子萌发和菌丝伸长[20]。另一个黄瓜基因CsWRKY50参与了黄瓜对霜霉病(Pseudoperonospora cubensis)的响应。CsWRKY50在黄瓜中的过表达增强了该植物对黄瓜霜霉病的抗性[21]。香蕉(Musa acuminata)中的2个WRKY基因(MaWRKY1和MaWRKY2)能够在病害反应中调节特定发病相关基因的表达来增加对炭疽菌的抗性[22]。在苹果抗病品种‘Sushuai’中,MdWRKY75d和MdWRKY75e基因的表达受到链格孢菌(Alternaria alternata)的诱导,其瞬时表达增强了转基因烟草和苹果对链格孢菌感染的抗性[23]。苹果MdWRKY15通过直接与MdICS1启动子结合来激活MdICS1的转录,增加了水杨酸(Salicylic acid, SA)的积累和疾病相关基因的表达,从而通过SA生物合成途径增强对轮纹病菌(Botryosphaeria dothidea)的抗性[24]。类似的研究表明,MdWRKY46通过激活MdPBS3.1的表达,从而依赖SA信号途径增强了苹果对轮纹病的抗性[25]。MdWRKY100是苹果炭疽病抗性的正调控因子,过表达MdWRKY100增加了苹果对炭疽菌的抗性,而利用RNAi沉默的转基因植株对炭疽菌更敏感[26]。苹果MdWRKY31能够在转录和翻译水平调控MdHIR4的表达,并依赖于SA信号途径增强转MdWRKY31基因拟南芥和烟草对病原菌PstDC3000的抗性[27]。在茶树(Camellia sinensis)中,一个属于Ⅱb亚组的CsWRKY14基因能够响应茶饼病菌(Exobasidium vexans),且CsWRKY14基因沉默的植物对茶饼病菌的敏感性增加[28]。此外,研究人员在WRKY基因参与番茄抗病应答方面也取得了一些进展。SlWRKY30基因受到青枯菌的强烈诱导,过表达该基因可降低番茄对青枯菌的易感性,并促进H2O2的积累和细胞坏死,表明SlWRKY30正向调节番茄对青枯菌的抗性[29]。在多毛番茄(Solanum habrochaites)中,ShWRKY81在抗白粉病株系LA1777中的表达水平高于易感株系。利用VIGS沉默该基因导致宿主对白粉菌的抵抗力下降,表明ShWRKY81正向调控多毛番茄对白粉菌的抗性[30]。研究人员在茄子(Solanum melongena)中克隆到与青枯病相关的SmWRKY65基因,该基因在根中的表达水平最高且响应青枯菌的诱导。利用VIGS技术沉默SmWRKY65基因的植株对青枯菌的感病性明显升高,表明该基因是调控茄子青枯病的正向调节子[31]。此外,研究人员在柑橘抗绿霉菌(Penicillium digitatum)方面取得进展,研究表明CsWRKY23、CsWRKY25和CsWRKY33参与了柑橘响应绿霉菌的调控。CsWRKY23能够对外源SA诱导产生应答,其在柑橘果皮中的瞬时过表达增强了对绿霉菌的抗性[32]。CsWRKY25的转录水平在感染绿霉菌的柑橘皮中上调,其过表达能增强柑橘对绿霉菌的抗性[33]。CsWRKY33通过结合自身启动子的W-box元件进行自我调节,其瞬时过表达显著增强了宿主对绿霉菌的抗性[34]。
除上述蔬菜瓜果类植物外,一些研究表明WRKY TF也参与了观赏花卉对生物胁迫应答的调控。在月季(Rosa chinensis)中,通过VIGS技术敲低RcWRKY41基因的表达导致转基因月季花瓣产生严重的灰霉病症状且其病斑大小显著增加,说明RcWRKY41基因对月季抗灰霉病有正向调控作用[35]。菊花(Chrysanthemum morifolium)CmWRKY15属于Ⅱa亚组WRKY TF,其表达受到链格孢菌的强烈诱导。与对照相比,过表达CmWRKY15增强了菊花对链格孢菌的易感性,表明CmWRKY15基因是菊花抗链格孢菌的负调控因子[36]。抗病基因CmWRKY15-1能直接调控CmNPR1(Non-expressor of pathogenesis-related genes 1)基因的表达,二者互作激活了下游发病机制相关基因的表达并通过SA途径增强对菊花白锈病菌(Puccinia horiana)的抗性[37]。从菊花品种‘Jinba’中克隆到的CmWRKY8-1基因参与了菊花对尖孢镰刀菌(Fusarium oxysporum)的响应,过表达CmWRKY8-1-VP64融合基因降低了菊花对尖孢镰刀菌的抗性[38]。
1.2 WRKY基因在园艺植物虫害中的作用植食性昆虫对植物的生存构成威胁,植物在生长过程中与植食性昆虫的相互作用及其调控机制是植物研究方面的重要问题。近年来的一些研究揭示了WRKY转录因子在园艺植物抵御虫害中的调控作用。菊花容易受到蚜虫的侵袭,研究发现菊花CmWRKY53基因表达受到蚜虫侵扰的诱导。与对照和CmWRKY53基因敲低植株相比,过表达CmWRKY53基因导致蚜虫数量显著增加,表明该基因是菊花抗蚜虫的负调节因子[39]。相反地,在菊花中过表达CmWRKY48基因显著降低了蚜虫的生长数量,说明CmWRKY48基因是菊花抗蚜虫的正向调节因子[40]。葡萄(Vitis vinifera)VvWRKY46基因能够响应葡萄根瘤蚜攻击,过表达VvWRKY46显著降低葡萄根瘤蚜的侵袭性,减缓若虫发育[41]。
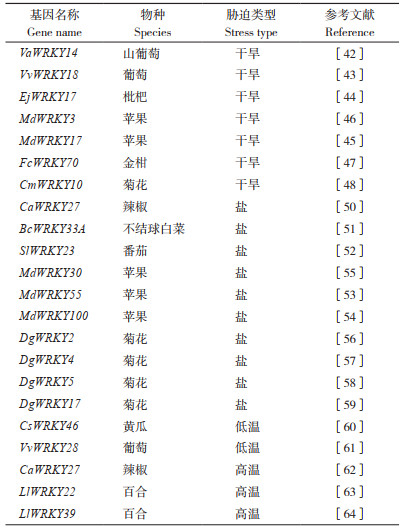
2 WRKY基因在园艺植物非生物胁迫应答中的作用 2.1 WRKY基因在园艺植物干旱胁迫应答中的作用干旱是常见的非生物胁迫之一,也是世界性的环境难题。干旱严重影响植物的分布区域、生长状态、产量和最终品质,深入研究园艺植物耐旱抗旱的分子机制十分必要。WRKY TF在园艺植物响应干旱胁迫中具有十分重要的作用(表 2)。山葡萄(Vitis amurensis)VaWRKY14基因编码1个Ⅱa亚组WRKY TF。在拟南芥中过表达VaWRKY14基因增强了转基因植株的耐旱能力。进一步的转录组分析表明,该基因可能通过调控胁迫相关基因的表达从而增强转基因拟南芥的抗旱性[42]。另一个在葡萄中克隆的基因VvWRKY18受到干旱和脱落酸(Abscisic acid, ABA)的诱导。VvWRKY18过表达的拟南芥叶片表现出气孔密度增加和失水率提高,导致对干旱的耐受性降低[43]。在拟南芥中过表达枇杷(Eriobotrya japonica)EjWRKY17基因,在干旱胁迫下可促进ABA介导的气孔关闭,显著上调转基因植株中ABA的生物合成和胁迫相关基因的表达,从而增强转基因拟南芥的耐旱性[44]。苹果MdWRKY17基因表达受到缺水的显著诱导。在中度干旱胁迫下,过表达MdWRKY17的转基因苹果植株叶绿素含量和光合作用速率显著高于对照,而在MdWRKY17敲低株系中则显著低于对照,表明该基因对苹果耐旱性有正向调控作用[45]。苹果MdWRKY3基因受到干旱胁迫诱导,能够提高转基因烟草的相对含水量等生理指标,增强转基因烟草的耐旱性[46]。FcWRKY70基因从金柑(Fortunella crassifolia)克隆获得,在烟草和柠檬中过表达该基因可增强相关转基因植物的耐寒能力。进一步分析表明FcWRKY70能够与精氨酸脱羧酶基因(FcADC)互作。在金桔中敲低FcWRKY70的表达降低FcADC的表达水平,表明FcWRKY70-FcADC模块可能参与了金桔的抗旱调控[47]。在菊花中,CmWRKY10的表达受到干旱的诱导。与野生型相比,过表达CmWRKY10提高了菊花对干旱胁迫的耐受性且促进了ABA信号通路相关基因的表达[48]。
|
2.2 WRKY基因在园艺植物盐胁迫应答中的作用
土壤盐渍化是植物生长发育和农业生产面临的重大环境问题。研究表明,预计到21世纪中叶,全球将有50% 以上的耕地受到土壤盐渍化的影响[49]。近年来的研究表明,WRKY TF在园艺植物盐胁迫应答以及耐盐调控方面发挥重要作用(表 2)。在辣椒中,CaWRKY27基因的表达受盐胁迫的诱导。过表达CaWRKY27基因的拟南芥和烟草对盐胁迫更加敏感,其根长和地上部分生长受到明显抑制。利用VIGS技术瞬时沉默CaWRKY27基因增强了辣椒对盐胁迫的抗性,表明该基因负调控辣椒对盐胁迫的抗性[50]。在受到盐胁迫的不结球白菜中,BcWRKY33A基因在根组织中显著表达。沉默BcWRKY33A基因的表达使不结球白菜对盐胁迫更加敏感[51]。在盐处理的番茄中,SlWRKY23基因表达水平升高。SlWRKY23在拟南芥中过表达增强了对NaCl的胁迫耐受性,并影响根系生长和侧根数[52]。在苹果中,MdWRKY55能够和MdNHX1形成复合体参与盐胁迫的调控。过表达MdWRKY55显著提高了苹果对盐胁迫的抗性[53]。MdWRKY100的表达受到miR156/SPL模块的激活和调控。过表达MdWRKY100显著增强了苹果对盐胁迫的抗性[54]。苹果WRKY Ⅱa亚组成员MdWRKY30的表达受到盐处理的诱导,其过表达可通过调控胁迫相关基因增强转基因苹果愈伤组织对盐胁迫的耐受性[55]。此外,菊花耐盐研究也取得了一些进展,DgWRKY2在菊花中的过表达增强了对盐胁迫的耐受性,该基因通过增强抗氧化和渗透调节,赋予转基因菊花耐盐性[56]。DgWRKY4受盐胁迫诱导,是一个响应盐胁迫的正调控基因,其通过提高光合能力、促进活性氧清除系统的运行、维持膜稳定性、增强渗透调节和上调胁迫相关基因的转录水平增加菊花对盐胁迫的耐受性[57]。DgWRKY5在菊花耐盐调控上发挥正向调控作用。在转DgWRKY5基因的菊花中,根长、鲜重、叶绿素含量和叶片气体交换参数等指标均优于野生对照[58]。与之相反,DgWRKY17在菊花耐盐调控上发挥负调控作用。在盐胁迫下,转DgWRKY17基因的菊花叶片中超氧化物歧化酶、过氧化物酶和脯氨酸含量显著低于对照,而叶片电导率则有所增加[59]。
2.3 WRKY基因在园艺植物温度胁迫应答中的作用温度胁迫属于重要的非生物胁迫类型,能够从生长发育、产量和地理分布范围方面对植物产生影响。近年来,全球气候的不稳定导致极端高温和低温事件频发。因此,探究植物对温度胁迫的响应机理具有十分重要的意义。研究表明,WRKY基因参与了园艺植物对温度胁迫的调控途径(表 2)。研究者从黄瓜中克隆并鉴定了CsWRKY46,其在冷胁迫和外源ABA处理下表达上调。与野生型相比,过表达CsWRKY46的转基因拟南芥在低温处理后具有更高的幼苗存活率。此外,CsWRKY46过表达系在种子萌发过程中对ABA敏感,表明CsWRKY46以ABA依赖的方式正向调节低温信号通路[60]。葡萄VvWRKY28基因在叶片中高表达,并受到低温胁迫的诱导。VvWRKY28过表达可影响转基因拟南芥丙二醛、叶绿素和脯氨酸含量等指标,从而提高转基因拟南芥对低温的耐受性[61]。在辣椒中,CaWRKY27的表达水平在高温胁迫期间升高,并在温度恢复期间持续表达。CaWRKY27过表达的拟南芥和烟草在高温胁迫下存活率降低,而CaWRKY27沉默的辣椒在高温胁迫下存活率升高。此外,在H2O2清除剂存在下,CaWRKY27的表达在热胁迫下受到抑制,而CaWRKY27沉默减少了H2O2在辣椒叶片中的积累。因此,CaWRKY27为H2O2介导高温应激反应的下游负调节因子[62]。在百合(Lilium longiflorum)中,LlWRKY22的表达被热刺激持续激活。同时,LlWRKY22在百合中的过表达提高了百合的耐热性,并激活了与热相关的LlDREB2基因的表达。LlWRKY22的沉默导致百合耐热性下降,说明LlWRKY22可能参与百合耐热性调控[63]。百合LlWRKY39基因也受到高温的诱导。LlWRKY39的异位表达提高了转基因百合和拟南芥的耐热性。此外,LlWRKY39、LlMBF1c、LlCaM3形成反馈调节通路来调控百合对高温的应答[64]。
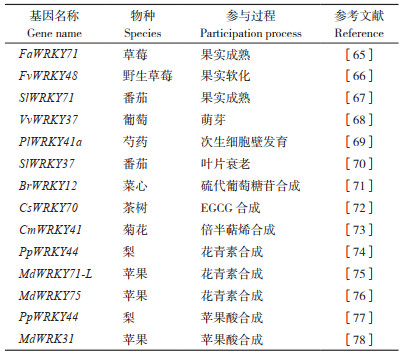
3 WRKY基因在园艺植物生长发育和物质合成中的作用 3.1 WRKY基因在园艺植物生长发育中的作用大量研究表明,WRKY TF广泛参与植物的各种生长发育调控(表 3)。在园艺植物果实成熟和器官衰老调控方面的相关研究也取得了一些进展。在草莓(Fragaria × ananassa)中,FaWRKY71在所有组织中都有表达,尤其是在全红果实中。此外,FaWRKY71促进类黄酮途径结构基因FaF3’H、FaLAR、FaANR及运输因子FaTT19和FaTT12的表达,并通过上调FaPG19和FaPG21的表达丰度来软化草莓的质地,促进草莓果实成熟[65]。在野生草莓(Fragaria vesca)中,过表达FvWRKY48导致果实中果胶细胞壁聚合物高半乳糖醛酸的降解加剧。此外,FvWRKY48能够结合FvPLA的启动子对其进行调控。FvPLA过表达果实的软化和果胶降解程度比对照果实更高,故FvWRKY48可能通过促进FvPLA的表达来调控野生草莓果胶降解和果实软化[66]。在番茄中,SlBRG3在Cys206和Cys212处发生过硫化,导致泛素化活性降低,与SlWRKY71转录物的相互作用减少,导致SlWRKY71与SlCAS1启动子的结合活性增加,导致其转录抑制,从而延迟番茄成熟[67]。休眠是植物在不合适的条件下暂时停止生长的常见生存策略。在葡萄中,VvWRKY37基因在休眠芽中高度表达。VvWRKY37的异位过表达显著延迟了转基因杨树植株的萌芽。VvWRKY37还抑制了ABA分解代谢基因CYP707As的表达,并提高内源ABA在转基因杨树中的积累,表明该基因可能通过ABA介导的信号通路调节萌芽[68]。在观赏植物芍药(Paeonia lactiflora)中,PlWRKY41a基因在茎组织中高度表达。此外,PlWRKY41a能与木聚糖内转葡糖基化酶/ 水解酶E4(PlXTH4)的启动子结合,并激活PlXTH4表达。过表达PlXTH4的烟草具有较厚的次生细胞壁,导致茎强度增加,而PlXTH4沉默的芍药具有较薄的次生细胞壁,茎强度降低,表明PlWRKY41a在芍药次生细胞壁发育过程中有重要调控作用[69]。番茄WRKY转录因子SlWRKY37能够对茉莉酸(Jasmonic acid, JA)和暗诱导的叶片衰老过程进行调控。SlWRKY37的敲除抑制了JA诱导和暗诱导的叶片衰老[70]。
|
3.2 WRKY基因在园艺植物代谢物合成过程中的作用
WRKY TF在园艺植物代谢物质合成调控中也具有重要作用(表 3)。硫代葡萄糖苷是一种含硫、氮的次生代谢产物,广泛存在于芸苔属植物中。在菜心(Brassica campestris)中,BrWRKY12可以直接与BrLOX4启动子区结合并激活其转录。沉默BrWRKY12降低了JA含量、下调了BrLOX4表达,并减少了硫代葡萄糖苷的积累[71]。表没食子儿茶素没食子酸酯(Epigallocatechin gallate, EGCG)是造成茶(Camellia sinensis)苦涩的重要因素。CsWRKY70是从茶中克隆的一个WRKY TF,能够结合EGCG生物合成关键酶基因CsLAR和CsUGT84A的启动子并调控其表达,从而影响EGCG在茶中的生物合成[72]。萜类化合物是挥发油的主要成分,在菊花中含量丰富。3-羟基-3-甲基戊二酰-CoA还原酶2(CmHMGR2)和法尼基焦磷酸合成酶2(CmFPPS2)在菊花萜类化合物的生物合成中起着关键作用。CmWRKY41可以通过GTGACA或CTGACG元件直接与CmHMGR2或CmFPPS2的启动子结合,并激活其表达以促进倍半萜烯(Sesquiterpenes)的生物合成[73]。花青素是人类饮食中抗氧化剂的来源,有助于水果着色。PpWRKY44基因是从梨(Pyrus L.)中鉴定到的一个光诱导WRKY基因家族成员。在梨叶片和果皮中瞬时过表达PpWRKY44显著增强了花青素的积累,而在梨果皮中沉默PpWRKY44则削弱了光对花青素积累的诱导[74]。MdWRKY75的过表达促进了苹果‘Orin’愈伤组织中花青素的积累。进一步研究表明,MdWRKY75主要通过与MYB转录因子MdMYB1的启动子结合来调控苹果花青素的积累[75]。此外,MdWRKY71-L的过表达促进了花青素在苹果愈伤组织中的积累。MdWRKY71-L主要通过与MdMYB1和MdUFGT的启动子相互作用来调控花青素积累[76]。苹果酸盐会影响果实酸度,在植物抗逆方面起至关重要的作用。在梨中,PpWRKY44通过直接结合苹果酸相关基因PpALMT9启动子上的W-box元件来激活其表达,从而参与盐诱导的苹果酸积累[77]。最近的研究表明,在苹果中存在MdWRKY31-MdERF72-MdALMT9基因模块,能够调节果实中苹果酸的积累[78]。
4 展望近年来,关于WRKY TF参与调控园艺植物生长发育以及抗逆机制的研究已取得长足进步,但相比在其他作物中的研究[79-80]依然不够丰富。越来越多的园艺植物进行了多类型的高通量测序,获得了大量的高质量基因组信息和基因表达信息,为园艺植物分子生物学的研究提供了有力支撑。此外,得益于生理学、化学遗传学和生物信息学等技术手段的应用,园艺植物WRKY TF介导的信号转导和调控的细节被逐步揭示,使得人们对园艺植物应对自身及外界刺激的复杂机制有了进一步了解。但相对于模式植物和主要农作物,园艺植物依然受转基因难度大、效率低的限制,许多研究只能在一些模式植物中进行基因过表达,且很难获得本体稳定转基因的株系,严重影响了园艺植物的分子育种研究。因此,加速园艺植物转基因技术的研究,建立高效、稳定的园艺植物转基因体系是亟待解决的热点问题。CRISPR/Cas9基因编辑技术已在许多植物中实现高效稳定的应用,未来应大力发展该技术在园艺植物中的研究和应用。
此外,WRKY家族作为植物中特有的转录因子家族,其在园艺植物中的数量相对庞大。现有对园艺植物WRKY TF的功能研究只是冰山一角,更重要的是,许多WRKY家族成员的功能并不单一。目前多数研究仅对WRKY基因进行单一方面的研究,并不能很好地揭示其更多的功能。因此,未来应该挖掘单一WRKY TF的多种功能,挖掘并鉴定一些具有主效、核心功能的WRKY基因。同时,由于全球环境不稳定,极端气候频发,园艺植物的生长发育和品质形成面临来自环境的巨大挑战。极端温度、干旱、盐碱和病虫害可能进一步影响甚至危害园艺植物的生长发育。因此,未来应该加速挖掘WRKY TF在园艺植物抗逆方面的重大作用,鉴定一些具有明显效用的WRKY候选基因,为园艺植物分子育种提供优良的遗传资源,加速园艺植物分子育种进程。
| [1] |
WANI S H, ANAND S, SINGH B, BOHRA A, JOSHI R. WRKY transcription factors and plant defense responses: Latest discoveries and future prospects[J]. Plant Cell Reports, 2021, 40(7): 1071-1085. DOI:10.1007/s00299-021-02691-8 |
| [2] |
LIU J J, EKRAMODDOULLAH A K. Identification and characterization of the WRKY transcription factor family in Pinus monticola[J]. Genome, 2009, 52(1): 77-88. DOI:10.1139/G08-106 |
| [3] |
张宇欣, 刘征, 张唐权, 郝园园, 成善汉, 汪志伟, 朱婕, 马武强, 包文龙. 空心菜WRKY基因家族成员鉴定与表达分析[J]. 分子植物育种, 2023, 21(1): 55-66. DOI:10.13271/j.mpb.021.000055 ZHANG Y X, LIU Z, ZHANG T Q, HAO Y Y, CHENG S H, WANG Z W, ZHU J, MA W Q, BAO W L. Genome-wide identification and expression analysis of WRKY gene family in water spinach (Ipomoea aquatica)[J]. Molecular Plant Breeding, 2023, 21(1): 55-66. DOI:10.13271/j.mpb.021.000055 |
| [4] |
MENG D, LI Y, BAI Y, LI M, CHENG L. Genome-wide identification and characterization of WRKY transcriptional factor family in apple and analysis of their responses to waterlogging and drought stress[J]. Plant Physiology and Biochemistry, 2016, 103: 71-83. DOI:10.1016/j.plaphy.2016.02.006 |
| [5] |
RUSHTON P J, SOMSSICH I E, RINGLER P, SHEN Q J. WRKY transcription factors[J]. Trends in Plant Science, 2010, 15(5): 247-258. DOI:10.1016/j.tplants.2010.02.006 |
| [6] |
CHEN L, SONG Y, LI S, ZHANG L, ZOU C, YU D. The role of WRKY transcription factors in plant abiotic stresses[J]. Biochimica et Biophysica Acta, 2010, 1819(2): 120-128. DOI:10.1016/j.bbagrm.2011.09.002 |
| [7] |
IFNAN KHAN M, ZHANG Y, LIU Z, HU J, LIU C, YANG S, HUSSAIN A, FURQAN A M, NOMAN A, SHEN L, XIA X, YANG F, GUAN D, HE S. CaWRKY40b in pepper acts as a negative regulator in response to Ralstonia solanacearum by directly modulating defense genes including CaWRKY40[J]. International Journal of Molecular Sciences, 2018, 19(5): 1403. DOI:10.3390/ijms19051403 |
| [8] |
DANG F F, WANG Y N, YU L, EULGEM T, LAI Y, LIU Z Q, WANG X, QIU A L, ZHANG T X, LIN J, CHEN Y S, GUAN D Y, CAI H Y, MOU S L, HE S L. CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection[J]. Plant, Cell and Environment, 2013, 36(4): 757-774. DOI:10.1111/pce.12011 |
| [9] |
CAI H, YANG S, YAN Y, XIAO Z, CHENG J, WU J, QIU A, LAI Y, MOU S, GUAN D, HUANG R, HE S. CaWRKY6 transcriptionally activates CaWRKY40, regulates Ralstonia solanacearum resistance, and confers high-temperature and high-humidity tolerance in pepper[J]. Journal of Experimental Botany, 2015, 66(11): 3163-3174. DOI:10.1093/jxb/erv125 |
| [10] |
DANG F, LIN J, CHEN Y, LI G X, GUAN D, ZHENG S J, HE S. A feedback loop between CaWRKY41 and H 2O2 coordinates the response to Ralstonia solanacearum and excess cadmium in pepper[J]. Journal of Experimental Botany, 2019, 70(5): 1581-1595. DOI:10.1093/jxb/erz006 |
| [11] |
RAFFEINER M, ÜSTÜN S, GUERRA T, SPINTI D, FITZNER M, SONNEWALD S, BALDERMANN S, BORNKE F. The Xanthomonas type-Ⅲ effector XopS stabilizes CaWRKY40a to regulate defense responses and stomatal immunity in pepper (Capsicum annuum)[J]. Plant Cell, 2022, 34(5): 1684-1708. DOI:10.1093/plcell/koac032 |
| [12] |
LI Y, MA X, XIAO L D, YU Y N, YAN H L, GONG Z H. CaWRKY50 acts as a negative regulator in response to Colletotrichum scovillei infection in pepper[J]. Plants, 2023, 12: 1962. DOI:10.3390/plants12101962 |
| [13] |
LI H, XU Y, XIAO Y, ZHU Z, XIE X, ZHAO H, WANG Y. Expression and functional analysis of two genes encoding transcription factors, VpWRKY1 and VpWRKY2, isolated from Chinese wild Vitis pseudoreticulata[J]. Planta, 2010, 232(6): 1325-1337. DOI:10.1007/s00425-010-1258-y |
| [14] |
WANG D, JIANG C, LIU W, WANG Y. The WRKY53 transcription factor enhances stilbene synthesis and disease resistance by interacting with MYB14 and MYB15 in Chinese wild grape[J]. Journal of Experimental Botany, 2020, 71(10): 3211-3226. DOI:10.1093/jxb/eraa097 |
| [15] |
WANG X, GUO R, TU M, WANG D, GUO C, WAN R, LI Z, WANG X. Ectopic expression of the wild grape WRKY transcription factor VqWRKY52 in Arabidopsis thaliana enhances resistance to the biotrophic pathogen powdery mildew but not to the necrotrophic pathogen Botrytis cinerea[J]. Frontiers in Plant Science, 2017, 8: 97. DOI:10.3389/fpls.2017.00097 |
| [16] |
YIN W, WANG X, LIU H, WANG Y, NOCKER S, TU M, FANG J, GUO J, LI Z, WANG X. Overexpression of VqWRKY31 enhances powdery mildew resistance in grapevine by promoting salicylic acid signaling and specific metabolite synthesis[J]. Horticulture Research, 2022, 9: 64. DOI:10.1093/hr/uhab064 |
| [17] |
张洁, 姜长岳, 王跃进. 中国野生毛葡萄转录因子VqWRKY6与VqbZIP1互作调控抗白粉病功能分析[J]. 中国农业科学, 2022, 55(23): 4626-4639. DOI:10.3864/j.issn.0578-1752.2022.23.005 ZHANG J, JIANG C Y, WANG Y J. functional analysis of the interaction between transcription factors VqWRKY6 and VqbZIP1 in regulating the resistance to powdery mildew in chinese wild Vitis quinquangularis[J]. Scientia Agricultura Sinica, 2022, 55(23): 4626-4639. DOI:10.3864/j.issn.0578-1752.2022.23.005 |
| [18] |
WANG Y, WANG X, FANG J, YIN W, YAN X, TU M, LIU H, ZhANG Z, LI Z, GAO M, LU H, WANG Y, WANG X. VqWRKY56 interacts with VqbZIPC22 in grapevine to promote proanthocyanidin biosynthesis and increase resistance to powdery mildew[J]. New Phytologist, 2023, 237(5): 1856-1875. DOI:10.1111/nph.18688 |
| [19] |
WEI W, CUI M Y, HU Y, GAO K, XIE Y G, JIANG Y, FENG J Y. Ectopic expression of FvWRKY42, a WRKY transcription factor from the diploid woodland strawberry (Fragaria vesca), enhances resistance to powdery mildew, improves osmotic stress resistance, and increases abscisic acid sensitivity in Arabidopsis[J]. Plant Science, 2018, 275: 60-74. DOI:10.1016/j.plantsci.2018.07.010 |
| [20] |
LIU M, ZHANG Q, WANG C, MENG T, WANG L, CHEN C, REN Z. CsWRKY10 mediates defence responses to Botrytis cinerea infection in Cucumis sativus[J]. Plant Science, 2020, 300: 110640. DOI:10.1016/j.plantsci.2020.110640 |
| [21] |
LUAN Q, CHEN C, LIU M, LI Q, WANG L, REN Z. CsWRKY50 mediates defense responses to Pseudoperonospora cubensis infection in Cucumis sativus[J]. Plant Science, 2019, 279: 59-69. DOI:10.1016/j.plantsci.2018.11.002 |
| [22] |
SHAN W, CHEN J Y, KUANG J F, LU W J. Banana fruit NAC transcription factor MaNAC5 cooperates with MaWRKYs to enhance the expression of pathogenesis-related genes against Colletotrichum musae[J]. Molecular Plant Pathology, 2016, 17(3): 330-338. DOI:10.1111/mpp.12281 |
| [23] |
HOU Y, YU X, CHEN W, WANG S, CAO L, GENG X, SUN C, QU S. Transcriptome sequencing, data-based screening, and functional investigation of MdWRKY75d and MdWRKY75e in disease-resistant apples[J]. Journal of Plant Interactions, 2021, 16: 1, 462-473. DOI:10.1080/17429145.2021.1981471 |
| [24] |
ZHAO X Y, QI C H, JIANG H, ZHONG M S, YOU C X, LI Y Y, HAO Y J. MdWRKY15 improves resistance of apple to Botryosphaeria dothidea via the salicylic acid-mediated pathway by directly binding the MdICS1 promoter[J]. Journal of Integrative Plant Biology, 2020, 62(4): 527-543. DOI:10.1111/jipb.12825 |
| [25] |
ZHAO X Y, QI C H, JIANG H, ZHONG M S, ZHAO Q, YOU C X, LI Y Y, HAO Y J. MdWRKY46-enhanced apple resistance to Botryosphaeria dothidea by activating the expression of MdPBS3.1 in the salicylic acid signaling pathway[J]. Molecular Plant Microbe Interact, 2019, 32(10): 1391-1401. DOI:10.1094/MPMI-03-19-0089-R |
| [26] |
ZHANG F, WANG F, YANG S, ZHANG Y, XUE H, WANG Y, YAN S, WANG Y, ZHANG Z, MA Y. MdWRKY100 encodes a group I WRKY transcription factor in Malus domestica that positively regulates resistance to Colletotrichum gloeosporioides infection[J]. Plant Science, 2019, 286: 68-77. DOI:10.1016/j.plantsci.2019.06.001 |
| [27] |
ZHAO X Y, QI C H, JIANG H, ZHONG M S, YOU C X, LI Y Y, HAO Y J. MdHIR4 transcription and translation levels associated with disease in apple are regulated by MdWRKY31[J]. Plant Molecular Biology, 2019, 101(1-2): 149-162. DOI:10.1007/s11103-019-00898-8 |
| [28] |
LIU S, ZHANG Q, GUAN C, WU D, ZHOU T, YU Y. Transcription factor WRKY14 mediates resistance of tea plants [ Camellia sinensis (L.) O. Kuntze]to blister blight[J]. Physiological and Molecular Plant Pathology, 2021, 115: 101667. DOI:10.1016/j.pmpp.2021.101667 |
| [29] |
DANG F, LIN J, LI Y, JIANG R, FANG Y, DING F, HE S, WANG Y. SlWRKY30 and SlWRKY81 synergistically modulate tomato immunity to Ralstonia solanacearum by directly regulating SlPR-STH2[J]. Horticulture Research, 2023, 10(5): 50. DOI:10.1093/hr/uhad050 |
| [30] |
WANG H, GONG W, WANG Y, MA Q. Contribution of a WRKY transcription factor, ShWRKY81, to powdery mildew resistance in wild tomato[J]. International Journal of Molecular Sciences, 2023, 24(3): 2583. DOI:10.3390/ijms24032583 |
| [31] |
刘开, 余炳伟, 李丹丹, 陈娜, 曹必好. 茄子SmWRKY65基因克隆及其对青枯病的抗性分析[J]. 广东农业科学, 2021, 48(3): 42-52. DOI:10.16768/j.issn.1004-874X.2021.03.006 LIU K, YU B W, LI D D, CHEN N, CAO B H. Cloning of SmWRKY65 gene and itsresistance to bacterial wilt in eggplant[J]. Guangdong Agricultural Sciences, 2021, 48(3): 42-52. DOI:10.16768/j.issn.1004-874X.2021.03.006 |
| [32] |
WANG W, LI T, CHEN Q, YAO S, ZENG K. Transcriptional regulatory mechanism of a variant transcription factor CsWRKY23 in citrus fruit resistance to Penicillium digitatum[J]. Food Chemistry, 2023, 413: 135573. DOI:10.1016/j.foodchem.2023.135573 |
| [33] |
WANG W, LI T, CHEN Q, YAO S, DENG L, ZENG K. CsWRKY25 improves resistance of citrus fruit to Penicillium digitatum via modulating reactive oxygen species production[J]. Frontiers in Plant Science, 2022, 12: 818198. DOI:10.3389/fpls.2021.818198 |
| [34] |
WANG W J, LI T, CHEN J L, ZHANG X, WEI L L, YAO S X, ZENG K F. A self-regulated transcription factor CsWRKY33 enhances resistance of citrus fruits to Penicillium digitatum[J]. Postharvest Biology and Technology, 2023, 198: 112267. DOI:10.1016/j.postharvbio.2023.112267 |
| [35] |
L I U X, L I D, ZH A NG S, XU Y, ZH A NG Z. G enome-wide characterization of the rose (Rosa chinensis) WRKY family and role of RcWRKY41 in gray mold resistance[J]. BMC Plant Biology, 2019, 19(1): 522. DOI:10.1186/s12870-019-2139-6 |
| [36] |
FAN Q, SONG A, XIN J, CHEN S, JIANG J, WANG Y, LI X, CHEN F. CmWRKY15 facilitates Alternaria tenuissima infection of chrysanthemum[J]. PLoS One, 2015, 10(11): e0143349. DOI:10.1371/journal.pone.0143349 |
| [37] |
GAO G, JIN R, LIU D, ZHANG X, SUN X, ZHU P, MAO H. CmWRKY15-1 promotes resistance to chrysanthemum white rust by regulating CmNPR1 expression[J]. Frontiers in Plant Science, 2022, 13: 865607. DOI:10.3389/fpls.2022.865607 |
| [38] |
MIAO W, GE L, WANG Y, LI S, SUN D, LIU Y, GUAN Z, CHEN S, FANG W, CHEN F, ZHAO S. Overexpression of CmWRKY8-1-VP64 fusion protein reduces resistance in response to Fusarium oxysporum by modulating the salicylic acid signaling pathway in Chrysanthemum morifolium[J]. International Journal of Molecular Sciences, 2023, 24(4): 3499. DOI:10.3390/ijms24043499 |
| [39] |
ZHANG W, GAO T, LI P, TIAN C, SONG A, JIANG J, GUAN Z, FANG W, CHEN F, CHEN S. Chrysanthemum CmWRKY53 negatively regulates the resistance of chrysanthemum to the aphid Macrosiphoniella sanborni[J]. Horticulture Research, 2020, 7(1): 109. DOI:10.1038/s41438-020-0334-0 |
| [40] |
LI P, SONG A, GAO C, JIANG J, CHEN S, FANG W, ZHANG F, CHEN F. The over-expression of a chrysanthemum WRKY transcription factor enhances aphid resistance[J]. Plant Physiology and Biochemistry, 2015, 95: 26-34. DOI:10.1016/j.plaphy.2015.07.002 |
| [41] |
WANG F P, ZHAO P P, ZHANG L, ZHAI H, DU Y P. Functional characterization of WRKY46 in grape and its putative role in the interaction between grape and phylloxera (Daktulosphaira vitifoliae)[J]. Horticulture Research, 2019, 6: 102. DOI:10.1038/s41438-019-0185-8 |
| [42] |
ZHANG L, CHENG J, SUN X, ZHAO T, LI M, WANG Q, LI S, XIN H. Overexpression of VaWRKY14 increases drought tolerance in Arabidopsis by modulating the expression of stress-related genes[J]. Plant Cell Reports, 2018, 37(8): 1159-1172. DOI:10.1007/s00299-018-2302-9 |
| [43] |
ZHANG L, ZHANG R, YE X, ZHENG X, TAN B, WANG W, LI Z, LI J, CHENG J, FENG J. Overexpressing VvWRKY18 from grapevine reduces the drought tolerance in Arabidopsis by increasing leaf stomatal density[J]. Journal of Plant Physiology, 2022, 275: 153741. DOI:10.1016/j.jplph.2022.153741 |
| [44] |
WANG D, CHEN Q, CHEN W, LIU X, XIA Y, GUO Q, JING D, LIANG G. A WRKY transcription factor, EjWRKY17, from Eriobotrya japonica enhances drought tolerance in transgenic Arabidopsis[J]. International Journal of Molecular Sciences, 2021, 22(11): 5593. DOI:10.3390/ijms22115593 |
| [45] |
SHAN D, WANG C, SONG H, BAI Y, ZHANG H, HU Z, WANG L, SHI K, ZHENG X, YAN T, SUN Y, ZHU Y, ZHANG T, ZHOU Z, GUO Y, KONG J. The MdMEK2-MdMPK6-MdWRKY17 pathway stabilizes chlorophyll levels by directly regulating MdSUFB in apple under drought stress[J]. Plant Journal, 2021, 108(3): 814-828. |
| [46] |
HAN D, ZHANG Z, DING H, WANG Y, LIU W, LI H, YANG G. Molecular cloning and functional analysis of MbWR KY3 involved in improved drought tolerance in transformed tobacco[J]. Journal of Plant Interactions, 2018, 13: 1, 329-337. DOI:10.1080/17429145.2018.1478994 |
| [47] |
GONG X, ZHANG J, HU J, WANG W, WU H, ZHANG Q, LIU J H. FcWRKY70, a WRKY protein of Fortunella crassifolia, functions in drought tolerance and modulates putrescine synthesis by regulating arginine decarboxylase gene[J]. Plant and Cell Environment, 2015, 38(11): 2248-2262. DOI:10.1111/pce.12539 |
| [48] |
JAFFAR M A, SONG A, FAHEEM M, CHEN S, JIANG J, LIU C, FAN Q, CHEN F. Involvement of CmWRKY10 in drought tolerance of chrysanthemum through the ABA-signaling pathway[J]. International Journal of Molecular Sciences, 2016, 17(5): 693. DOI:10.3390/ijms17050693 |
| [49] |
谢颖悦, 王琦, 王春平, 周冰玉, 周宇, 刘芬, 孙翔宇. 植物响应盐胁迫的机制研究进展[J]. 激光生物学报, 2022, 31(05): 398-403. DOI:10.3969/j.issn.1007-7146.2022.05.003 XIE Y Y, WANG Q, WANG C P, ZHOU B Y, ZHOU Y, LIU F, SUN X Y. Progress of studies on the mechanism of plant response to salt stress[J]. Acta Laser Biology Sinica, 2022, 31(5): 398-403. DOI:10.3969/j.issn.1007-7146.2022.05.003 |
| [50] |
LIN J, DANG F, CHEN Y, GUAN D, HE S. CaWRKY27 negatively regulates salt and osmotic stress responses in pepper[J]. Plant Physiology and Biochemistry, 2019, 145: 43-51. DOI:10.1016/j.plaphy.2019.08.013 |
| [51] |
WANG H, LI Z, REN H, ZHANG C, XIAO D, LI Y, HOU X, LIU T. Regulatory interaction of BcWRKY33A and BcHSFA4A promotes salt tolerance in non-heading Chinese cabbage [ Brassica campestris (syn. Brassica rapa) ssp. chinensis][J]. Horticulture Research, 2022, 9: 113. DOI:10.1093/hr/uhac113 |
| [52] |
SINGH D, DEBNATH P, SANE A P, SANE V A. Tomato (Solanum lycopersicum) WRKY23 enhances salt and osmotic stress tolerance by modulating the ethylene and auxin pathways in transgenic Arabidopsis[J]. Plant Physiology and Biochemistry, 2023, 195: 330-340. DOI:10.1016/j.plaphy.2023.01.002 |
| [53] |
SU M, WANG S, LIU W, YANG M, ZHANG Z, WANG N, CHEN X. Interaction between MdWRKY55 and MdNAC17-L enhances salt tolerance in apple by activating MdNHX1 expression[J]. Plant Science, 2022, 320: 111282. DOI:10.1016/j.plantsci.2022.111282 |
| [54] |
MA Y, XUE H, ZHANG F, JIANG Q, YANG S, YUE P, WANG F, ZHANG Y, LI L, HE P, ZHANG Z. The miR156/SPL module regulates apple salt stress tolerance by activating MdWRKY100 expression[J]. Plant Biotechnology Journal, 2021, 19(2): 311-323. DOI:10.1111/pbi.13464 |
| [55] |
DONG Q, ZHENG W, DUAN D, HUANG D, WANG Q, LIU C, LI C, GONG X, LI C, MAO K, MA F. MdWRKY30, a group Ⅱa WRKY gene from apple, confers tolerance to salinity and osmotic stresses in transgenic apple callus and Arabidopsis seedlings[J]. Plant Science, 2020, 299: 110611. DOI:10.1016/j.plantsci.2020.110611 |
| [56] |
HE L, W U Y H, ZHAO Q, WANG B, L IU Q L, ZHANG L. Chrysanthemum DgWRKY2 gene enhances tolerance to salt stress in transgenic chrysanthemum[J]. International Journal of Molecular Sciences, 2018, 19(7): 2062. DOI:10.3390/ijms19072062 |
| [57] |
WANG K, WU Y H, TIAN X Q, BAI Z Y, LIANG Q Y, LIU Q L, PAN Y Z, ZHANG L, JIANG B B. Overexpression of DgWRKY4 enhances salt tolerance in chrysanthemum seedlings[J]. Frontiers in Plant Science, 2017, 8: 1592. DOI:10.3389/fpls.2017.01592 |
| [58] |
LIANG Q Y, WU Y H, WANG K, BAI Z Y, LIU Q L, PAN Y Z, ZHANG L, JIANG B B. Chrysanthemum WRKY gene DgWRKY5 enhances tolerance to salt stress in transgenic chrysanthemum[J]. Scientific Reports, 2017, 7(1): 4799. DOI:10.1038/s41598-017-05170-x |
| [59] |
LI P, SONG A, GAO C, WANG L, WANG Y, SUN J, JIANG J, CHEN F, CHEN S. Chrysanthemum WRKY gene CmWRKY17 negatively regulates salt stress tolerance in transgenic chrysanthemum and Arabidopsis plants[J]. Plant Cell Reports, 2015, 34(8): 1365-1378. DOI:10.1007/s00299-015-1793-x |
| [60] |
ZHANG Y, YU H, YANG X, LI Q, LING J, WANG H, GU X, HUANG S, JIANG W. CsWRKY46, a WRKY transcription factor from cucumber, confers cold resistance in transgenic-plant by regulating a set of coldstress responsive genes in an ABA-dependent manner[J]. Plant Physiology and Biochemistry, 2016, 108: 478-487. DOI:10.1016/j.plaphy.2016.08.013 |
| [61] |
LIU W, LIANG X, CAI W, WANG H, LIU X, CHENG L, SONG P, LUO G, HAN D. Isolation and functional analysis of VvWRKY28, a Vitis vinifera WRKY transcription factor gene, with functions in tolerance to cold and salt stress in transgenic Arabidopsis thaliana[J]. International Journal of Molecular Sciences, 2022, 23(21): 13418. DOI:10.3390/ijms232113418 |
| [62] |
DANG F, LIN J, XUE B, CHEN Y, GUAN D, WANG Y, HE S. CaWRKY27 negatively regulates H2O2-mediated thermotolerance in pepper (Capsicum annuum)[J]. Frontiers in Plant Science, 2018, 9: 1633. DOI:10.3389/fpls.2018.01633 |
| [63] |
WU Z, LI T, CAO X, ZHANG D, TENG N. Lily WRKY factor LlWRKY22 promotes thermotolerance through autoactivation and activation of LlDREB2B[J]. Horticulture Research, 2022, 9: 186. DOI:10.1093/hr/uhac186 |
| [64] |
DING L, WU Z, TENG R, XU S, CAO X, YUAN G, ZHANG D, TENG N. LlWRKY39 is involved in thermotolerance by activating LlMBF1c and interacting with LlCaM3 in lily (Lilium longifl orum)[J]. Horticulture Research, 2021, 8(1): 36. DOI:10.1038/s41438-021-00473-7 |
| [65] |
YUE M, JIANG L, ZHANG N, ZHANG L, LIU Y, WANG Y, LI M, LIN Y, ZHANG Y, ZHANG Y, LUO Y, WANG X, CHEN Q, TANG H. Importance of FaWRKY71 in strawberry (Fragaria×ananassa) fruit ripening[J]. International Journal of Molecular Sciences, 2022, 23(20): 12483. DOI:10.3390/ijms232012483 |
| [66] |
ZHANG W W, ZHAO S Q, GU S, CAO X Y, ZHANG Y, NIU J F, LIU L, LI A R, JIA W S, QI B X, XING Y. FvWRKY48 binds to the pectate lyase FvPLA promoter to control fruit softening in Fragaria vesca[J]. Plant Physiology, 2022, 189(2): 1037-1049. DOI:10.1093/plphys/kiac091 |
| [67] |
SUN C, YAO G F, LI L X, LI T T, ZHAO Y Q, HU K D, ZHANG C, ZHANG H. E3 ligase BRG3 persulfidation delays tomato ripening by reducing ubiquitination of the repressor WRKY71[J]. Plant Physiology, 2023, 192(1): 616-632. DOI:10.1093/plphys/kiad070 |
| [68] |
WANG F P, ZHAO P P, ZHANG L, ZHAI H, ABID M, DU Y P. The VvWRKY37 regulates bud break in grape vine through ABA-mediated signaling pathways[J]. Frontiers in Plant Science, 2022, 13: 929892. DOI:10.3389/fpls.2022.929892 |
| [69] |
TANG Y, LU L, HUANG X, ZHAO D, TAO J. The herbaceous peony transcription factor WRKY41a promotes secondary cell wall thickening to enhance stem strength[J]. Plant Physiology, 2023, 191(1): 428-445. DOI:10.1093/plphys/kiac507 |
| [70] |
WANG Z, GAO M, LI Y, ZHANG J, SU H, CAO M, LIU Z, ZHANG X, ZHAO B, GUO Y D, ZHANG N. The transcription factor SlWRKY37 positively regulates jasmonic acid- and dark-induced leaf senescence in tomato[J]. Journal of Experimental Botany, 2022, 73(18): 6207-6225. DOI:10.1093/jxb/erac258 |
| [71] |
YUE L, KANG Y, LI Y, KANG D, ZHONG M, CHAI X, GUO J, YANG X. 1-Methylcyclopropene promotes glucosinolate biosynthesis through BrWRKY12 mediated jasmonic acid biosynthesis in postharvest flowering Chinese cabbage[J]. Postharvest Biology and Technology, 2023, 203: 112415. DOI:10.1016/j.postharvbio.2023.112415 |
| [72] |
SONG X, HUANG X, Li Q, LIN H, BAI S, ZHU M, LI J, WANG K. The WRKY transcription factor CsWRKY70 regulates EGCG biosynthesis by affecting CsLAR and CsUGT84A expressions in tea leaves (Camellia sinensis)[J]. Horticulturae, 2023, 9: 120. DOI:10.3390/horticulturae9010120 |
| [73] |
HU Y, SONG A, GUAN Z, ZHANG X, SUN H, WANG Y, YU Q, FU X, FANG W, CHEN F. CmWRKY41 activates CmHMGR2 and CmFPPS2 to positively regulate sesquiterpenes synthesis in Chrysanthemum morifolium[J]. Plant Physiology and Biochemistry, 2023, 196: 821-829. DOI:10.1016/j.plaphy.2023.02.036 |
| [74] |
ALABD A, AHMAD M, ZHANG X, GAO Y, PENG L, ZHANG L, NI J, BAI S, TENG Y. Light-responsive transcription factor PpWRKY44 induces anthocyanin accumulation by regulating PpMYB10 expression in pear[J]. Horticulture Research, 2022, 9: 199. DOI:10.1093/hr/uhac199 |
| [75] |
SU M, ZUO W, WANG Y, LIU W, ZHANG Z, WANG N, CHEN X. The WKRY transcription factor MdWRKY75 regulates anthocyanins accumulation in apples (Malus domestica)[J]. Functional Plant Biology, 2022, 49(9): 799-809. DOI:10.1071/FP21146 |
| [76] |
SU M, WANG S, LI C, ZHANG Z, WANG N, LI B, CHEN X. Ultraviolet-B-induced MdWRKY71-L expression regulates anthocyanin synthesis in apple[J]. Environmental and Experimental Botany, 2022, 201: 105000. DOI:10.1016/j.envexpbot.2022.105000 |
| [77] |
ALABD A, CHENG H, AHMAD M, WU X, PENG L, WANG L, YANG S, BAI S, NI J, TENG Y. ABRE-BINDING FACTOR3-WRKY DNABINDING PROTEIN44 module promotes salinity-induced malate accumulation in pear[J]. Plant Physiology, 2023, 192(3): 1982-1996. DOI:10.1093/plphys/kiad168 |
| [78] |
WANG J H, GU K D, ZHANG Q Y, YU J Q, WANG C K, YOU C X, CHENG L, HU D G. Ethylene inhibits malate accumulation in apple by transcriptional repression of aluminum-activated malate transporter 9 via the WRKY31-ERF72 network[J]. New Phytologist, 2023, 239(3): 1014-1034. DOI:10.1111/nph.18795 |
| [79] |
徐磊, 刘洋. 高粱组Ⅲ WRKY转录因子对干旱胁迫的表达分析[J]. 广东农业科学, 2021, 48(2): 11-16. DOI:10.16768/j.issn.1004-874X.2021.02.002 XU L, LIU Y. Expression analysis of group Ⅲ WRKY transcription factors in sorghum under drought st ress[J]. Guangdong Agricultural Sciences, 2021, 48(2): 11-16. DOI:10.16768/j.issn.1004-874X.2021.02.002 |
| [80] |
杜英俊, 朱鹏锦, 钟云婕, 唐秀观, 何江, 宋奇琦, 欧景莉, 叶维雁. 菠萝蜜响应低温胁迫的转录组分析[J]. 中国南方果树, 2023, 52(4): 37-44, 50. DOI:10.13938/j.issn.1007-1431.20220652 DU Y J, ZHU P J, ZHONG Y J, TANG X G, HE J, SONG Q Q, OU J L, YE W Y. Transcriptome analysis of jackfruit in response to low temperature stress[J]. South China Fruits, 2023, 52(4): 37-44, 50. DOI:10.13938/j.issn.1007-1431.20220652 |
(责任编辑 陈丽娥)
 2023, Vol. 50
2023, Vol. 50