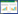文章信息
基金项目
- 国家现代农业产业技术体系- 新疆西甜瓜产业技术体系项目(CARS-25)
作者简介
- 耿新丽(1975—), 女, 硕士, 研究员, 研究方向为甜瓜贮藏与运输, E-mail: 89069899@qq.com.
通讯作者
- 千春录(1982—), 男, 博士, 副教授, 研究方向为果蔬品质形成生理及控制, E-mail: clqian@yzu.edu.cn.
文章历史
- 收稿日期:2023-09-07
2. 南京农业大学食品科学与技术学院, 江苏 南京 210095;
3. 塔里木大学食品科学与工程学院, 新疆 阿拉尔 843300;
4. 扬州大学食品科学与工程学院, 江苏 扬州 225127
2. College of Food Science and Technology, Nanjing Agricultural University, Nanjing 210095, China;
3. College of Food Science and Engineering, Tarim University, Aral 843300, China;
4. College of Food Science and Engineering, Yangzhou University, Yangzhou 225127, China
Muskmelon (Cucumis melo L.), a plant of Cucurbitaceae, is one of the fruits most favored by consumers. So far, it has been widely and increasingly planted around the world. Moreover, with the increasing requirements for new varieties and good fruit quality by both consumers and the market, traditional outdoor planting has been far from enough to meet their demands[1-4]. Since the end of last century, the development of protected cultivation of muskmelons has resulted in gradual industrialization of cultivation and production of muskmelons all the year round. Therefore, protected cultivation has been widely accepted by many muskmelon growers. However, in cultivating and planting, except for selection of muskmelon varieties cultivated in appropriate facilities, attention should be paid to the effects of environmental factors (illumination, temperature and humidity) in the facilities on muskmelon quality. Light intensity has effects on not only plant photosynthesis but also yield and quality. In cultivating and planting muskmelons, many researchers focused more on the relationship between illumination and photosynthesis in planting muskmelon plants than on the effect of low-light on muskmelon quality[5-6]. It has been proved that low-light stress could inhibit the accumulation of saccharides in leaves and fruits, but the responses of different varieties of muskmelons to low-light stress was different[7].
In order to reveal the effects of low-light stress on fruit quality of main muskmelon varieties in Xinjiang, China, three widely-planted muskmelon varieties in Shanshan, Xinjiang were selected in this study. Muskmelon plants at different growth stages were treated under different light intensities. Then, muskmelons were harvested at different stages. Contents of fruit carbohydrates, carotenoids and chlorophyll of the muskmelons were determined to identify effects of light intensity on physiological characteristics of fruit development and coloration of the muskmelons, with a view to providing a certain theoretical reference for the quality and efficient cultivation of muskmelons, and contributing to selection and further extensive cultivation of lowlight-tolerant muskmelon varieties.
1 Materials and methods 1.1 Experimental materialsThe experiments were conducted on pilot farm of the Research Institute of Grape, Melon and Fruits in Xinjiang Urgur Autonomous Region, Shanshan, Xinjiang in 2021—2023. Three varieties Xizhoumi No.1, Zaohuanghou and Jiashi of muskmelon [Cucumis melo L. var. saccharinus Naud. Xizhoumi No.1, Cucumis melo L. var. saccharinus Naud. Zaohuanghou and Cucumis melo L. var. saccharinus Naud. Jiashi (hereinafter referred to as Xizhoumi No.1, Zaohuanghou and Jiashi muskmelon, respectively)] were selected as materilas. All seeds of the three varieties of muskmelon were purchased from Shanshan County Seed Company. According to production practice of muskmelon, all the three muskmelon varieties were assigned to three groups: (1) the control, with a transmittance of 100% light intensity; (2) planting under a single-layer black sun shading net, with 42% light intensity of the control group; and (3) planting under a black sun shading net with a layer of white insect-proof screen, with 23% light intensity of the control group. Guard rows were set up around each group. Management modes of plants in experimental plots were in accordance with those in other common fields.
Sun shading nets with a net height of 1 m were set up 1 week before the blooming of muskmelons. They were labeled at day of muskmelon blooming, ensuring their orderly and uniform growth. Muskmelon plants with normal ovary development were sampled at 0, 3, 7, 14, 21, 35, 40, 60 d after blooming, respectively. For each variety, 10 muskmelons were randomly harvested for each treatment group at each stage. All treatments and measurements were triply replicated. An average of 50 seedlings were involved in ecch treatment.
1.2 Determination of carbohydrates1.2.1 Determination of starch content The starch content of muskmelon was measured by the chromogenic iodine method[8], from 7 d to 35 d after blooming.
1.2.2 Determination of reducing sugar content Muskmelon fruit tissue (1.0 g) was ground and homogenized with a small amount of distilled water in a mortar, and the pulp was filled to volume 25 mL. The total homogeneous liquid was kept warm in a water bath at 80 oC for 30 min. After removal and cooling, the extract was filtered. All the filtrates were mixed and used as reducing sugar and sucrose extracts.
The reducing sugar content of muskmelon was measured as described with some modification[9], from 7 d to 35 d after blooming. The 2.0 mL of reducing sugar extract was mixed with 1.5 mL of 3, 5-dinitrosalicylic acid (0.6%), and the mixture was heated in boiling water bath for 5 min, then removed and immediately cooled in cold water to room temperature. The reaction mixture was filled to volume 25 mL with distilled water. The absorbance at 540 nm was measured by a spectrophotometer and quantified by standard curve.
1.2.3 Determination of sucrose content The sucrose content of muskmelon was measured as described with some modification[10], from 7 d to 35 d after blooming. 0.1 mL of the sucrose extract was added to 0.1 mL of 30% KOH, and placed in a boiling water bath for 10 min, then cooled to room temperature. 3 mL of anthrone reagent (150 mg of anthrone dissolved in 100 mL of 76% sulfuric acid) was added, and the temperature was kept at 40 ℃ for 15 min. The absorbance at 620 nm was measured and quantified by standard curve.
1.3 Determination of carotenoidsThe carotenoids content of muskmelon was measured as described with some modification[11], from 20 d to 60 d after blooming. The muskmelons were peeled and 2 small pieces were took diagonally from each fruit. Sample pieces were roughly chopped together. Samples (100 g) were immediately mashed in a high-speed tissue masher for 3 min. Then all pulp was wrapped with gauze and squeezed for juice. 20 mL of juice was filtered through funnel with ashless filter, then filtrate was put in a separatory funnel. 20 mL of extraction agent 〔Chloroform-methanol: 2/1 (V/V)〕 was put in the funnel and mixed with filtered juice, shaked and waited for the delamination of liquid, then the lower organic phase was collected as carotenoids extract. The absorbance of carotenoids extract at 440 nm was measured with a spectrophotometer. The carotenoid content was calculated with the following formulas:

|
The determination of chlorophyll of muskmelon was based on the method of Li et al[12], from 20 d to 60 d after blooming.
1.5 Data processing and analysisSPSS 24.0 statistical software was used for analysis of variance, and Turkey method was used to determine the significance of difference. P < 0.05 was indicated as significant difference.
2 Results and analysis 2.1 Responses of carbohydrates in muskmelon to different light intensities2.1.1 Response of starch content in muskmelon to different light intensities As shown in Fig. 1, starch content in muskmelon increased from 7 d to 35 d after full blooming. Compared with the control, starch content was highest in Xizhoumi No.1 (10.835%), followed by Zaohuanghou (9.755%) and Jiashi muskmelon (8.342%). General trends in the three muskmelons treated under different light intensities showed that starch content was lower at each of the five fruit development stages as the decrease of transmittance than that in the control group. Namely, compared with control, the fruit starch content in the low light with 23% transmittance decreased by 32.3%, 32.63% and 41.08% in Xizhoumi No.1, Zaohuanghou and Jiashi muskmelons during maturity (35 d), respectively, indicative of a certain difference in the effects of low-light stress on fruit starch contents of the three muskmelons, in which Jiashi muskmelon was most heavily affected by low-light and showed the most starch loss.
|
| Fig. 1 Responses of starch contents in different muskmelon varieties to different light intensities |
2.1.2 Response of sucrose content in muskmelon to different light intensities As shown in Fig. 2, fruit sucrose content varied in different parts of the muskmelon and began to increase from 7 d to 35 d after blooming. The sucrose contents were 4.856%, 5.604% and 5.686% in the pedicel, pulp and areola of the mature fruit of Xizhoumi No.1 (35 d, Fig. 2A), respectively〔2.355%, 2.814% and 3.03% in the corresponding parts of Zaohuanghou (Fig. 2C) and 5.319%, 5.787% and 7.637% in the corresponding parts of Jiashi (Fig. 2B), respectively〕. Therefore, sucrose contents were higher in the pulp and areola and lowest in the pedicle of muskmelon fruit. In other words, the pulp and the areola tasted sweet but the pedicel tasted bad. General trends of sucrose content in the three muskmelons treated under different light intensities showed that sucrose content was lower at each of the five fruit development stages than that in the control group as transmittance decreased. Namely, compared with control, the fruit sucrose contents in the low light with 23% transmittance decreased by 29.87% and 23.55% in the pulp and areola of Xizhoumi No.1 (Fig. 2A), 25.09% and 21.22% in the pulp and areola of Zaohuanghou (Fig. 2C), and 31.64% and 32.39% in the pulp and areola of Jiashi (Fig. 2B) during maturity (35 d), respectively, indicative of a certain difference in the effects of low-light stress on fruit sucrose contents of the three muskmelon varieties, in which Jiashi muskmelon was most heavily affected and showed the most sucrose loss (Fig. 2B).

|
| Fig. 2 Responses of sucrose contents in different muskmelon varieties to different light intensities |
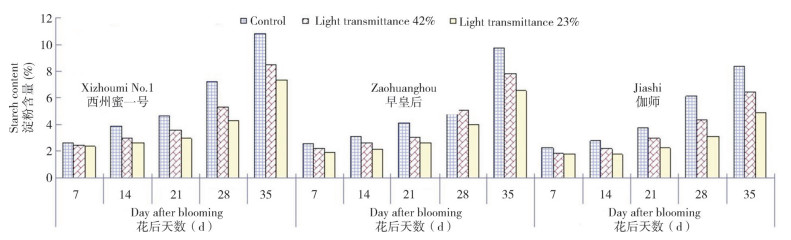
2.1.3 Changes in fruit reducing sugar content in different parts of muskmelon under different light intensities As shown in Fig. 3, fruit reducing sugar content varied in different parts of the muskmelon and began to increase from 7 d to 35 d after blooming. Compared with control, the reducing sugar contents were 0.938%, 0.961% and 0.985% in the pedicel, pulp and areola of the mature fruit (35 d) of Xizhoumi No.1 (Fig. 3C), respectively 〔0.904%, 0.936% and 0.945% in the corresponding parts of Zaohuanghou (Fig. 3B) and 0.862%, 1.069% and 0.994% in the corresponding parts of Jiashi (Fig. 3A), respectively〕, and there was no significant (P > 0.05) difference in reducing sugar content between pedicel, pulp and areola of the muskmelon. General trends in the three muskmelons treated under different light intensities showed that reducing sugar content was lower at each of the five fruit development stages than that in the control group as transmittance decreased. Namely, compared with control, the fruit reducing sugar contents in the low light with 23% transmittance decreased by 26.23%, 23.1% and 28.02% in the pedicel, pulp and areola of Xizhoumi No.1 (Fig. 3C), 20.24%, 11.43% and 13.54% in the pedicel, pulp and areola of Zaohuanghou (Fig. 3B), and 28.89%, 39.1% and 33.1% in the pedicel, pulp and areola of Jiashi (Fig. 3A) during maturity, respectively, indicative of a certain difference in the effects of low-light stress on fruit reducing sugar contents of the three muskmelons, in which Jiashi muskmelon was most heavily affected and showed the most reducing sugar loss (Fig. 3A).
|
| Fig. 3 Responses of reducing sugar contents in different muskmelon varieties to different light intensities |
2.2 Changes in fruit carotenoid content of muskmelon under different light intensities
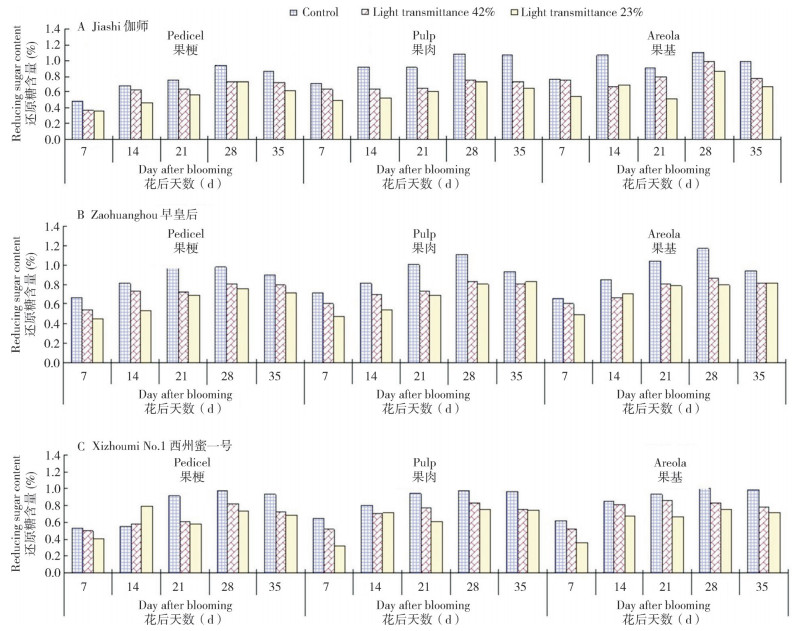
As shown in Fig. 4, fruit carotenoid contents of different muskmelons varied in three picking periods and tended to increase first and decrease afterwards. The fruit carotenoid content of the muskmelon was maximal when picked at 40 d after blooming, and carotenoid contents were 4.559, 3.120 and 3.185 mg/g in Xizhoumi No.1, Zaohuanghou and Jiashi, respectively. General trends in the three muskmelons treated under different light intensities showed that carotenoid content was higher in each of the three picking periods than that in the control group as transmittance decreased. At 40 d after blooming, compared with control, the fruit carotenoid content in the low light with 23% transmittance increased by 19.47%, 54.74% and 55.20% in Xizhoumi No.1, Zaohuanghou and Jiashi, respectively, indicative of a certain difference in effects of low-light stress on fruit carotenoid contents of the three muskmelons, in which Jiashi muskmelon was most heavily affected and showed the most carotenoid increase (Fig. 4B).
|
| Fig. 4 Responses of fruit carotenoid contents in different muskmelon varieties to different light intensities |
2.3 Changes in pericarp chlorophyll content of muskmelon under different light intensities
As shown in Fig. 5, pericarp chlorophyll contents of different muskmelons varied in three picking periods and tended to increase first and decrease afterwards. The pericarp chlorophyll content of the muskmelon was maximal when picked at 40 d after blooming, the pericarp chlorophyll contents were 2.149, 1.530 and 1.492 mg/g in Xizhoumi No.1, Zaohuanghou and Jiashi, respectively. The chlorophyll content declined at 60 d after blooming, and the contents were 0.472, 1.282 and 0.368 mg/g in Xizhoumi No.1, Zaohuanghou and Jiashi, respectively. Compared with control, general trends in the three muskmelons treated under different light intensities showed that chlorophyll content washigher in each of the three picking periods than that in the control group as transmittance decreased, suggesting that poor coloration may occur in muskmelon fruits under the low-light stress so that they looked as dark green as young fruits when ripe, thereby affected the fruit marketability severely. At 40 d after blooming, compared with control, the pericarp chlorophyll contents in the low light with 23% transmittance increased by 32.99%, 47.19% and 56.97% in Xizhoumi No.1, Zaohuanghou and Jiashi muskmelon, respectively. At 60 d after blooming, compared with control, the pericarp chlorophyll contents in the low light with 23% transmittance increased by 13.98%, 21.06% and 31.79% in Xizhoumi No.1, Zaohuanghou and Jiashi, respectively, indicative of a certain difference in the effects of low-light stress on pericarp chlorophyll contents of the three muskmelons, in which Jiashi muskmelon was most heavily affected and showed the most chlorophyll increase.

|
| Fig. 5 Responses of pericarp chlorophyll contents in different muskmelon varieties to different light intensities |
3 Discussion
Among numerous environmental factors influencing plant growth and development, the light has the greatest effect on plants. How the light affects plant growth and development is mainly manifested in two aspects: One is to affect plant photosynthesis, and the other is to regulate plant growth and development. Every plant species has its own demand for illumination. If the illumination provided is not sufficient or the duration of light intensity is below the light saturation point, plant will grow under low-light stress [4, 13].
In general, each photosynthetic parameter of the plant will change under low-light stress. First, low light may have an effect on plant photosynthetic efficiency. Many studies have shown that within a certain range of light intensity, plant photosynthetic efficiency will decrease with the decrease of light intensity. Second, it will affect chlorophyll content and its photosynthetic characteristics. Chloroplast is a major organelle where higher plants carry out photosynthesis. Effects of illumination on chloroplast content and its structure also varies with species. It has been reported that chloroplast development is affected by regulatory effects of light factors. The chlorophyll content markedly increased through different light treatments compared with control, and continually increased with the decrease of light intensity[14]. This study used three widely-planted muskmelon varieties (Xizhoumi No.1, Zaohuanghou and Jiashi) in Shanshan, Xinjiang as test materials to investigate changes in pericarp chlorophyll content in low light during different sampling periods. There was a certain difference in the effects of low-light stress on pericarp chlorophyll content of the three muskmelons. Particularly, Jiashi muskmelon was most heavily affected, and its pericarp chlorophyll content in low light with 23% transmittance increased by 31.79% compared with control, with most increase and poorest low-light tolerance.
Light controls plant cell differentiation and changes in structures and functions, and finally results in construction of tissues and organs. Division and differentiation of plant organs, yield formation and quality are resulted from solar radiation and plant photosynthesis. The role of low-light stress in plant ecological and environmental factors is partly due to decrease of solar radiation and land surface temperature, thereby affecting the external environment of plant growth. Light factor is of particular importance in the process of vegetative growth to reproductive growth for plants. Plant dry matter accumulation will be suppressed, affecting the florescence, pollination and fruit setting of the plant, and thus yield and quality under low-light stress. Muskmelon is fragrant, sweet and nutritious, rich in sugar, starch, minerals and vitamins. Sugar content is a major factor measuring muskmelon quality and influencing its flavor. Different illuminations will have impacts on accumulation of nutritional components of the muskmelon, like sugar, and thus its fruit quality[15-16]. The normality of fruit growth and development and the fruit quality mainly depend on accumulation, distribution and transformation of sugar in different parts of the fruit, and there is a certain difference in sugar accumulation among different varieties, even among different parts of the same fruit, which partly reflects a difference in endoplasmic factor of the fruit. The low light inhibited its dry matter accumulation, resulting in a slow process of reproductive growth[17]. Shading treatment led to poorly-colored fruits and decrease in soluble matter content and fruit firmness[14]. This study found a difference in carbohydrate content in the pedicle, pulp and areola of the muskmelon fruit of three muskmelon varieties, with higher content in the pulp; it was also found a certain difference in the effects of low-light stress on fruit carbohydrate contents in different muskmelon varieties, in which Jiashi muskmelon was most heavily affected and showed the worst tolerance.
In addition, light facilitates the synthesis of fruit carotenoids, particularly the accumulation of β-cryptoxanthin, mainly due to the fact that light affects the formation of β-carotenoids in the form of environmental signals. Light intensity changed the ratio of LYC-b to LYC-e m RNAs in arabidopsis [Arabidopsis thaliana (L.) Heynh.] and tomato (Solanum lycopersicum L.) [2]. The expression of PSY and PDS genes was upregulated by illumination[1]. Through the study, it was found that, by comparison of carotenoid content in several turfgrass species in natural light, there was a significant difference in the ratio of β-carotene and its derivatives to α-carotene and its derivatives. Therefore, the ratio of carotenoid content to content of its pigments could be regarded as an indicator reflecting the shade tolerance of turfgrass. Low carotenoid content was also confirmed in the shaded tangerine fruits and light-colored fruits[3, 18]. In this study, it was concluded that, in low light with 23% transmittance, Jiashi muskmelon showed highest increase in carotenoid content, which increased by 55.20% compared with control, indicative of a certain difference in the effects of low-light stress on carotenoid contents of the three muskmelons, in which Jiashi muskmelon was most heavily affected with poor low-light tolerance.
4 ConclusionThe contents of fruit starch, sucrose and reducing sugar all increased continuously during muskmelon fruit development and the highest contents exhibited at mature stage (35 d). There was higher sucrose content in the pulp and areola of the fruit than that in pedicle, but no difference in reducing sugar content. The muskmelon carotenoid and chlorophyll content increased during fruit development but decceased at full ripe stage (60 d). Low-light stress significantly reduced the carbohydrate content, increased carotenoid and chlorophyll contents, thus reduced the commercial value of muskmelon, and the lower the light transmittance, the more severe it became; under the transmittance 23% treatment, the contents of starch, sucrose and reducing sugar in Jiashi muskmelon reduced up to 41.08%, 32.39%, and 39.1%, while contents of carotene and chlorophyll increased by 55.20% and 31.79%, respectively. Different varieties of muskmelon showed different responses to low-light stress, and Jiashi muskmelon was most sensitive to low-light stress and experienced the greatest quality loss under the stress.
| [1] |
BOHNE F, LINDEN H. Regulation of carotenoid biosynthesis genes in response to light in Chlamydomonas reinhardtii[J]. Biochimica et Biophysica Acta-Gene Structure and Expression, 2002, 1579(1): 26-34. DOI:10.1016/S0167-4781(02)00500-6 |
| [2] |
HIRSCHBERG J. Carotenoid biosynthesis in flowering plants[J]. Current Opinion in Plant Biology, 2001, 4(3): 210-218. DOI:10.1016/S1369-5266(00)00163-1 |
| [3] |
RODRIGO M J, MARCOS J F, ZACARIAS L. Biochemical and molecular analysis of carotenoid biosynthesis in flavedo of orange (Citrus sinensis L.) during fruit development and maturation[J]. Journal of Agricultural and Food Chemistry, 2004, 52(22): 6724-6731. DOI:10.1021/jf049607f |
| [4] |
SEKHAR S, PANDA D, KUMAR J, MOHANTY N, BISWAL M, BAIG M J, KUMAR A, UMAKANTA N, SAMANTARAY S, PRADHAN S K, SHAWN B P, SWAIN P, BEHERA L. Comparative transcriptome profiling of low light tolerant and sensitive rice varieties induced by low light stress at active tillering stage[J]. Scientifi c Reports, 2019, 9: 5753. DOI:10.1038/s41598-019-42170-5 |
| [5] |
LI Y, XIN G F, WEI M, SHI Q H, YANG F J, WANG X F. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities[J]. Scientia Horticulturae, 2017, 225: 490-497. DOI:10.1016/j.scienta.2017.07.053 |
| [6] |
ZHU H F, LI X F, ZHAI W, LIU Y, GAO Q Q, LIU J P, REN L, CHEN H Y, ZHU Y Y. Effects of low light on photosynthetic properties, antioxidant enzyme activity, and anthocyanin accumulation in purple pak-choi (Brassica campestris ssp. Chinensis Makino)[J]. Plos One, 2017, 12(6): e0179305. DOI:10.1371/journal.pone.0179305 |
| [7] |
YANG L Y, CHEN J J, SUN X M, LI J X, CHEN N L. Inhibition of sucrose and galactosyl-sucrose oligosaccharide metabolism in leaves and fruits of melon (Cucumis melo L.) under low light stress[J]. Scientia Horticulturae, 2018, 244: 343-351. DOI:10.1016/j.scienta.2018.09.001 |
| [8] |
WOODARD H Q. Colorimetric determination of iodine by the starch-iodine reaction[J]. Industrial and Engineering Chemistry, 1934, 6(5): 331-333. DOI:10.1021/ac50091a014 |
| [9] |
CHEKROUD Z, DJERRAB L, ROUABHIA A, DEMS M A, ATAILIA L, DJAZY F, SMADI M A. Valorisation of date fruits by-products for production of biopolymer polyhydroxybutyrate (PHB) using the bacterial strain Bacillus paramycoides[J]. Italian Journal of Food Science, 2022, 34(3): 48-58. DOI:10.15586/ijfs.v34i3.2236 |
| [10] |
SILVA F V, SOUZA G B, NOGUEIRA A R A. Use of yeast crude extract for sequential injection determination of carbohydrates[J]. Analytical Letters, 2001, 34(8): 1377-1388. DOI:10.1081/AL-100104161 |
| [11] |
ADJEI M O, MA J, LUO R X, HUANG J F, ZHAO Z C, WANG Y Y, GAO A P. Tra nscr iptome a na lyses revea led chil ling response genes in mango (Mangifera indica L. cv. Keitt) leaf[J]. Journal of Plant Interactions, 2023, 18(1): 2172226. DOI:10.1080/17429145.2023.2172226 |
| [12] |
LI C, SUO J, XUAN L, DING M, ZHANG H, SONG L, YING Y. Bamboo shoot-lignification delay by melatonin during low temperature storage[J]. Postharvest Biology and Technology, 2019, 156: 110933. DOI:10.1016/j.postharvbio.2019.110933 |
| [13] |
刘科, 何爱斌, 苑涛涛, 田小海, 张运波. 弱光胁迫对超级杂交稻产量和干物质积累的影响[J]. 广东农业科学, 2014, 41(18): 20-27. DOI:10.16768/j.issn.1004-874X.2014.18.024 LIU K, HE A B, YUAN T T, TIAN X H, ZHANG Y B. Efects of weak light stress on grain yield and dry mater accumulation of super hybrid rice[J]. Guangdong Agricultural Sciences, 2014, 41(18): 20-23. DOI:10.16768/j.issn.1004-874X.2014.18.024 |
| [14] |
BAWA G, CHEN G P, SHI J Y, PING C, FENG L Y, PU T, YANG H, CHEN H, KAI S, HU Y, LIAN B, BIN C, XIAO T, MEMON S U R, YANG F, YONG T W, LIU J, LIU W G, WANG X C, YANG W Y. Further insights into how low-light signaling delays leaf senescence in soybean under high-temperature[J]. Environment and Experimental Botany, 2021, 188: 104516. DOI:10.1016/j.envexpbot.2021.104516 |
| [15] |
LIU Y X, PAN T, TANG Y Y, ZHUANG Y, LIU Z J, LI P H, LI H, HUANG W Z, TU S B, REN G J, WANG T, WANG S H. Proteomic analysis of rice subjected to low light stress and overexpression of OsGAPB increases the stress tolerance[J]. Rice, 2020, 13(1): 30. DOI:10.1186/s12284-020-00390-8 |
| [16] |
朱子超, 李贤勇, 王楚桃, 蒋刚, 李顺武, 欧阳杰, 黄乾龙, 熊英, 何永歆. 生育后期弱光胁迫对不同类型水稻的影响[J]. 广东农业科学, 2014, 41(16): 1-3, 36. DOI:10.16768/j.issn.1004-874X.2014.16.021 ZHU Z C, LI X Y, WANG C T, JIANG G, LI S W, OUYANG J, HUANG Q L, XIONG Y, HE Y X. Efects of shading during late growth stage on different rice type[J]. Guangdong Agricultural Sciences, 2014, 41(16): 1-3, 36. DOI:10.16768/j.issn.1004-874X.2014.16.021 |
| [17] |
ISLAM M S, MATSUI T, YOSHIDA Y. Carbohydrate content and activities of sucrose synthase, sucrose phosphate synthase and acid invertase in different tomato cultivars during fruit development[J]. Scientia Horticulturae, 1996, 65: 125-136. |
| [18] |
黄子锋, 王凤兰, 方展奋, 张昭其. 弱光和1-MCP处理对盆栽月季贮运品质的影响[J]. 广东农业科学, 2018, 45(1): 34-38. DOI:10.16768/j.issn.1004-874X.2018.01.006 HUANG Z F, WANG F L, FANG Z F, ZHANG Z Q. Effects of weak light and 1-MCP treatments on storage qualities of Rosa chinensis[J]. Guangdong Agricultural Sciences, 2018, 45(1): 34-38. DOI:10.16768/j.issn.1004-874X.2018.01.006 |
(责任编辑 陈丽娥)
 2024, Vol. 51
2024, Vol. 51