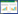文章信息
基金项目
- 广东省区域联合基金-重点项目(2019B1515120088);广东省农村科技特派员项目(2020);广东省农业科学院人才培养项目(R2022PY-QF002)
作者简介
- 卓梦霞(1999—),女,土家族,在读硕士生,研究方向为植物资源保护与利用,E-mail:2045824988@qq.com.
通讯作者
- 胡位荣(1966—),男,博士,教授,研究方向为果蔬采后生物学,E-mail:weironghu@163.com.
文章历史
- 收稿日期:2023-08-10
2. 广东省农业科学院果树研究所/农业农村部南亚热带果树生物学与遗传资源利用重点实验室/广东省热带亚热带果树研究重点实验室,广东 广州 510640
2. Institute of Fruit Tree Research, Guangdong Academy of Agricultural Sciences/Key Laboratory of Biology and Genetic Resource Utilization of Southern Subtropical Fruit Trees, Ministry of Agriculture and Rual Aff airs/Guangdong Key Laboratory of Tropical and Subtropical Fruit Tree Research, Guangzhou 510640, China
真菌毒素是植物病原真菌产生的对植物有害的次生代谢物,主要由镰刀菌属(Fusarium spp.)、链格孢霉属(Alternaria spp.)、曲霉属(Aspergillus spp.)、青霉属(Penicillium spp.)等真菌产生,其中部分真菌毒素会严重威胁人和动物的健康[1],摄入真菌毒素会造成急性或慢性中毒,引起肠道、肝脏、肾脏毒性、生殖毒性、免疫毒性、细胞毒性及致癌性[2-3]。镰刀菌属真菌可侵染包括小麦、棉花、水稻、番茄、香蕉、茄子等在内的100多种植物,是世界上最重要的植物病原菌之一。目前对镰刀菌的致病机理研究主要集中在破坏植物细胞结构和分泌致病毒素2方面,前者主要是病原菌在植物维管束内大量定殖,破坏维管束功能,同时产生各种细胞壁降解酶(如纤维素酶、果胶酶、β-葡萄糖苷酶),并利用其降解产物(果胶或胼胝质等物质)堵塞植物维管束,造成水分和营养缺失,导致植物萎蔫、死亡[4-5];后者分泌的毒素是镰刀菌在侵染植物过程中的关键致病因子之一,能促进病原菌入侵,降低寄主的防御能力。当病原菌入侵植物后,由于毒素浓度不断增加,引起植物细胞结构和外部形态的改变,导致植物防御能力进一步下降,加速病原菌的侵染[6]。
针对病原菌的侵染,植物已进化出多种应对策略。防御基因的表达和防御酶的激活是植物应对病原菌的一种策略,同时,植物还利用解毒酶、转运蛋白和自身产生的一些次生代谢物质去应对病原菌产生的毒素。镰刀菌属毒素是极其重要的一类真菌毒素,因其种类繁多、性质结构不一、毒性较强,能加速植物病害反应进程,是近年来真菌毒素领域的研究热点。本文立足于病原菌和植物互作,就镰刀菌属主要毒素的生物合成途径、毒性机理、毒素引起植物的生理变化及植物应对病原菌毒素的策略展开综述,以期为镰刀菌的致病机理研究及其防治新思路提供参考。
1 镰刀菌属真菌毒素的种类、生物合成途径及其毒性机理镰刀菌属真菌毒素主要包括单端孢霉烯族毒素(Trichothecenes,Tri)、玉米赤霉烯酮类毒素(Zearalenone,ZEN)、伏马菌素(Fumonisins,FBs)、镰刀菌酸(Fusaric acid,FSA)、串珠镰刀菌酸(Moniliformin,MON)、白僵菌素(Beauvericin,BEA)和恩镰孢菌素(Enniatins,ENNs)等[7]。这些毒素由一系列不同的真菌产生,具有不同的化学结构和毒性(表 1),了解真菌毒素的生物合成及毒性机理对深入解析病原菌的致病机理至关重要。
|
1.1 单端孢霉烯族毒素
单端孢霉烯族毒素(Tri)是一类主要由镰刀菌等丝状真菌产生的倍半萜类物质,分为A、B、C、D四类,其中最常见且毒性最强的是A型和B型毒素。A型毒素主要包括:T-2毒素、HT-2毒素;B型毒素包括:雪腐镰刀菌烯醇(Nivalenol,NIV)、脱氧雪腐镰刀菌烯醇(Deoxynivalenol,DON)及其乙酰化产物3-乙酰脱氧雪腐镰刀菌烯醇(3-acetyldeoxynivalenol,3-ADON)和15-乙酰脱氧雪腐镰刀菌烯醇(15-acetyldeoxynivalenol,15-ADON)[8]。Tri由15个Tri基因编码,其生物合成至少涉及3个基因家族:Tri5基因簇、Tri101基因簇和Tri1~Tri16基因簇[9]。其中,Tri5编码单端孢霉二烯合酶,催化反式法尼基焦磷酸环化形成单端孢霉二烯,Tri4编码细胞色素P450单加氧酶,参与加氧、羟化、异构化反应[10-11],Tri1~Tri16编码的官能团负责环化、酯化反应,并以此区分A型和B型毒素,最终形成不同结构的的单端孢霉烯族毒素[12-13]。Tri能够抑制DNA、RNA和蛋白质的合成,抑制细胞分化,改变细胞膜通透性和扰乱线粒体的正常功能[14-15]。但是其结构复杂性的增加并不一定会引起毒素对植物毒性的改变,其毒性主要根据寄主的不同而有所差异。如T-2毒素和4, 15-乙酰脱氧雪腐镰刀菌烯醇(4, 15-acetyldeoxynivalenol,4, 15-ADON)对拟南芥和小麦的毒性较高,DON和3-ADON的毒性却较低[16]。T-2毒素和DON分别能诱导拟南芥和小麦的活性氧(Reactive oxygen species,ROS)积累和细胞坏死[17]。也有报道称DON是禾谷镰刀菌侵染小麦并扩散所必需的,产DON毒素能力的丧失会导致镰刀菌不能从接种部位向其他部位扩散[4, 18]。
1.2 玉米赤霉烯酮类毒素玉米赤霉烯酮类毒素(ZEN)是一种非甾体类雌激素毒素,具有十分稳定的化学结构,常作为污染物存在于粮食作物和饲料中。由于ZEN及其代谢物的结构与内源性雌激素17β-雌二醇类似,因此ZEN能够引起家畜高雌性激素综合症,扰乱雌性激素的平衡,造成生殖障碍[19-20]。同时ZEN具有细胞毒性,通过诱导ROS积累和细胞膜电位的丧失而加速细胞凋亡[21],且有研究表明其能通过PI3K-AKT-mTOR和MAPK信号通路刺激鸡颗粒细胞的自噬[22]。ZEN在大麦中能转化为玉米赤霉烯酮-14-葡萄糖苷(ZEN-14-G)和玉米赤霉烯酮-16-葡萄糖苷(ZEN-16-G),可能有助于镰刀菌在植物中增殖[23]。
1.3 伏马菌素伏马菌素(FBs)是一类由轮枝镰刀菌(F. verticillioides)、层出镰刀菌(F. proliferatum)和藤仓镰刀菌(F. fujikuroi)等真菌产生的聚酮类化合物。根据结构差异,可以分为A、B、C和P类,其中最常见的是B类,而FB1是毒性最强且分布最广泛的。伏马菌素的生物合成由FUM基因簇调控,首先由FUM1编码的聚酮合成酶催化形成毒素的基本骨架,随后经FUM6、FUM2和FUM15编码的细胞色素P450单加氧酶、FUM7编码的脱氢酶、FUM8编码的羟胺合成酶、FUM9编码的双加氧酶、FUM13编码的还原酶、FUM10和FUM16编码的脂肪酰辅酶A合成酶等修饰形成[24-25]。这种毒素可导致马脑白质软化、猪肺水肿、人类食道癌和肝癌等疾病[26]。同时,该毒素具有植物毒性,0.5 μmol/L FB1处理便可造成枸杞根、茎、叶生长受抑制,进而促进植株的衰老[27];FB1处理会显著抑制玉米幼苗主根和侧根的生长[28];引发植物的超敏反应(Hypersensitive response,HR),同时通过促进ROS的积累造成叶绿体蛋白降解,引起细胞膜脂质过氧化,导致植物细胞死亡[29]。由于FB1的化学结构和鞘脂代谢通路中的鞘氨醇相似,二者互相竞争神经酰胺合酶的活性部位,扰乱神经鞘脂的生物合成,破坏鞘脂代谢和信号传导,引起植物细胞膜脂的破坏,进而导致植物的程序性细胞死亡(Programmed cell death,PCD)[30-31]。Niehaus等[32]研究表明,藤仓镰刀菌菌株B14产生的FB1可能会抵消由赤霉素所引起的水稻恶苗病常见症状,而导致植株发育迟缓或枯萎。也有研究报道,FB1处理棉花幼苗会产生类似于大丽轮枝菌侵染后的症状,也会加速层出镰刀菌侵染采后香蕉的速率[33-34]。
1.4 镰刀菌酸镰刀菌酸是(FSA)第一个从受感染寄主植物中分离得到的真菌毒素,也是最早报道的由尖孢镰刀菌番茄专化型引起番茄枯萎病的真菌毒素之一[35]。FSA是一种非寄主特异性毒素,在低浓度(低于10-6 mol/L)时能激活植物对于生物胁迫的防御反应,但高浓度时则能加速番茄叶片中ROS的积累,降低植物抗氧化酶的活性,抑制ATP的合成,诱导细胞死亡。此外,FSA可能是铜、铁、锌等金属离子的螯合剂,以此对番茄植物产生强烈的毒性作用[35-36]。FSA的生物合成由12个FUB基因组成的基因簇共同编码,其中9个基因是尖孢镰刀菌和轮枝镰刀菌中合成FSA所必需的,但缺乏FSA并不影响尖孢镰刀菌对仙人掌和轮枝镰刀菌对玉米幼苗的致病力[37]。Liu等[38]研究表明FSA能作为先锋分子早于尖孢镰刀菌出现在香蕉假茎中,并通过干扰寄主的线粒体功能,引起ROS爆发,导致香蕉细胞发生HR并形成坏死,协助病原菌扩散。
1.5 串珠镰刀菌素串珠镰刀菌素(MON)是由Cole等[39]首次在串珠镰刀菌(F. moniliforme)的培养物中分离得到的一种水溶性真菌毒素,对动植物均具有毒性,亦会引起动物和人体心肌损伤。MON是一种硫胺素焦磷酸(TPP)依赖酶的抑制剂,其化学结构与丙酮酸相似,可通过抑制线粒体丙酮酸和a-酮戊二酸的氧化来抑制三羧酸循环,阻止能量代谢途径[40-42]。此外,MON具有急性毒性、免疫毒性和生殖毒性,且对不同物种之间的毒性存在差异,鸟类似乎比啮齿动物对此更加敏感。串珠镰刀菌素能抑制小麦胚芽鞘的生长,造成烟草叶片卷曲肥大,引起玉米植株黄化或坏死,也能显著抑制玉米愈伤组织的生长[39, 43-44]。有研究发现在感染果腐病的苹果中能够检测到MON,该毒素可能对苹果产品的绿色加工产生威胁[45]。
1.6 白僵菌素和恩镰孢菌素白僵菌素(BEA)和恩镰孢菌素(ENNs)的化学结构和合成过程十分相似,均是由核糖体合酶合成的环状六肽[46]。BEA是一种有效的生物活性化合物,具有抗菌、杀虫、抗癌的作用[41, 47-48]。BEA的生物合成由4个基因组成的基因簇共同编码,最早在藤仓镰刀菌(F. fujikuroi)中鉴定得到。其中,Bea1编码非核糖体肽合成酶(Nonribosomal peptide synthetases,NRPs);Bea2、Bea3和Bea4分别编码具有运输和调节功能的蛋白质[49-50]。BEA可以作为离子载体结合在细胞膜上,形成阳离子选择通道,影响细胞内离子平衡;也可以通过刺激ROS的产生而发挥其细胞毒性作用[42]。此外,BEA具有遗传毒性,通过改变线粒体膜电位导致DNA片段化,诱导细胞凋亡[51-52]。作为一种真菌毒素,BEA可能在植物病原菌的致病过程中发挥重要作用。Paciolla等[53]研究表明,50 μmol/L BEA可能通过破坏番茄抗坏血酸系统从而诱导原生质体死亡。李春雨等[54]首次在香蕉枯萎病菌中发现了BEA,离体实验也证明BEA可以使香蕉假茎腐烂。
ENNs具有和BEA类似的抗菌、杀虫、抗肿瘤等生物功能[55-56],且也能作为一种离子载体,结合阳离子形成亲脂复合体,通过细胞膜运输改变细胞内的离子浓度,导致细胞功能紊乱[57],通过引起线粒体功能障碍和ROS的产生,导致细胞死亡[58]。燕麦镰刀菌(F. avenaceum)中ENNs合成基因(esyn)的敲除,显著降低了其对马铃薯块茎的致病力[59]。Eranthodi等[60]研究揭示,虽然燕麦镰刀菌中产生的ENNs并不会加重硬粒小麦赤霉病或豌豆根腐病的病情严重程度,但其可能会增加与燕麦镰刀菌共生的其他病原菌的致病力。Ederli等[61]研究表明,ENNs能显著促进小麦细胞死亡。此外,ENNs虽然可抑制禾谷镰刀菌的生长,但也促进燕麦镰刀菌的生长,这可能有助于燕麦镰刀菌的致病。
2 镰刀菌属真菌侵染过程及毒素引起的生理变化真菌毒素是病原菌成功侵染植物的毒力因子之一,在镰刀菌属病原菌侵染过程中发挥着重要作用。镰刀菌与植物的互作复杂多样,均伴随着生理状态的改变。
2.1 镰刀菌属真菌的侵染过程植物与病原菌相互作用的过程复杂且多样。病原菌侵染植物常常包括以下4个过程:病原菌识别寄主、附着在寄主表面、穿透寄主表皮细胞并产生致病因子、菌丝定殖并在外部引起发病症状[62-63]。巴西蕉感染香蕉枯萎病菌热带4号生理小种(Fusarium oxysporum f. sp. cubense tropical race 4,Foc TR4)后,会出现叶片枯黄、假茎开裂、维管束堵塞等症状[64]。Li等[65]利用激光共聚焦显微镜观察绿色荧光蛋白(Green fluorescent protein,GFP)标记的Foc侵染香蕉的过程中发现,Foc TR4可以穿透巴西蕉细胞间隙和根表皮细胞壁,并能在根茎维管束中大量定殖,而Foc race1却只能通过伤口进入;Foc穿透根部细胞并在根茎维管束中定殖可能是其成功侵染巴西蕉并导致相关病症的关键。而植物细胞壁的穿透是通过真菌分泌的植物细胞壁降解酶如纤维素酶、果胶酶、木聚糖酶和脂肪酶等作用实现的[66]。这些物质可以作为病原体相关分子模式(Pathogen-associated molecular patterns,PAMPs),触发植物的免疫反应[7, 67]。
2.2 镰刀菌属真菌毒素引起植物细胞的生理变化病原菌侵染寄主后产生的毒素会激活寄主自身抗病相关代谢活动。真菌毒素主要作用于寄主植物的细胞质膜、线粒体和叶绿体[68]。一部分真菌毒素会攻击膜脂,而另一部分会以膜蛋白为靶点,但无论结合位点在哪里,都会引起细胞膜透性改变,导致细胞膜功能紊乱,细胞膜透性发生改变几乎是各种敏感植株对毒素的一个普遍反应。同时,真菌毒素可使寄主植物的细胞质膜发生破裂而失水,从而影响寄主植物的水分代谢,导致植株出现萎蔫。陈慧杰等[69]研究表明,菊花枯萎病菌粗毒素FSA可抑制切花菊幼苗根、茎的正常生长,增加幼苗根系的细胞膜透性及根系组织中可溶性糖、脯氨酸和丙二醛(Malondialdehyde,MDA)的含量,导致植物水分运输受阻,枯萎致死。但可以在短时间内提高根系保护酶过氧化物酶(Peroxidase,POD)、多酚氧化酶(Polyphenol oxidase,PPO)和苯丙氨酸解氨酶(Phenylalanine ammonia lyase,PAL)活性,产生积极的防御反应,保护细胞不受伤害。
在对植物和病原菌互作的研究中,人们对酶的活性变化展开了广泛的研究。大部分研究表明,FSA处理植物后,POD、PPO和PAL的活性均有所升高[70-71]。一般认为,POD在植物抗病性中的作用与细胞壁的加固有关,之所以有这种现象,是POD可进一步催化木质素单体脱氢聚合成木质素大分子物质,加固细胞壁,阻止毒素的识别和病原菌的生长扩散[72]。PPO可将酚类物质氧化为醌类物质,醌类化合物又可破坏氧化还原电位,能抑制病原菌生长发育所需的磷酸化酶和转氨酶,抑制病原菌产生毒素和包括在果胶酶和纤维素酶在内的细胞壁降解酶,阻止病原菌进一步入侵[73]。PAL是苯丙烷代谢途径的关键酶和限速酶,参与植物木质素、酚类、类黄酮类等次生物质的合成代谢,其中许多物质具有抑制病原菌生长和毒素产生的活性,与植物的防御反应密切相关[74]。
赵潇璨等[75]研究表明,用5 ng/mL DON处理马铃薯块茎,其抗氧化酶和防御酶活性升高,同时DON作为激发子可诱导马铃薯块茎中内源激素水杨酸(Salicylic acid,SA)、茉莉酸(Jasmonic acid,JA)和乙烯(Ethylene,ETH)的含量增加, 植物系统抗性调控基因NPR1的表达量上调,诱导了马铃薯的系统获得抗性(Systemic acquired resistance,SAR);说明低浓度或适当浓度的毒素可能会诱导植物自身抗性的激活,抵御病原菌入侵。此外,Luo等[76]研究表明,禾谷镰刀菌侵染后的小麦为了缓解病原菌产生的DON对自身生长的不利影响,通常会增加包括超氧化物歧化酶(Superoxide dismutase,SOD)、过氧化氢酶(Catalase,CAT)和抗坏血酸过氧化物酶(Ascorbate peroxidase,APX)等在内的抗氧化酶活性。而这些物质可以作为植物组织抗性的评判标准之一,这表明镰刀菌毒素能够提高植物抗病相关酶的活性,诱导寄主的自身抗性。
3 植物防御真菌毒素的策略植物和病原菌经过长期的斗争,植物针对病原菌毒素已经进化出至少两种应对策略,即化学修饰和转运[7]。化学修饰是将有毒的化合物经过各种化学反应转化为无毒的化合物。植物利用自身防御机制将病原菌毒素转化为真菌毒素衍生物,这种衍生物又称“隐蔽型真菌毒素”[77]。
3.1 解毒酶参与植物脱毒化学修饰往往需要解毒酶的参与,包括谷胱甘肽S转移酶(Glutathione S-transferases,GSTs)、UDP-糖基转移酶(Uridine diphosphate glycosyltransferases,UGTs)等在内的解毒酶在植物应对病原菌毒素上扮演着重要的角色。Wang等[78]发现小麦Fhb7基因编码GSTs,其可能参与到DON的解毒过程,通过去环氧化作用将DON转化为低毒的谷胱甘肽(Glutathione,GSH)复合物(DON-GSH),减轻对小麦细胞的毒性,增加小麦对禾谷镰刀菌的抗性。Chetouhi等[79]研究禾谷镰刀菌侵染小麦过程中的基因表达量发现,多数参与抑制细胞死亡的基因表达上调,而参与凋亡诱导因子的基因表达下调,以维持正常的细胞功能;并认为DON在禾谷镰刀菌侵染初期并不是必需的,但病原菌在侵染过程中产生的DON可能有利于增加菌落的大小,通过诱导H2O2的产生,刺激植物的PCD,大量积累DON将有利于真菌将植物的营养模式转变为坏死营养,表明小麦中的PCD可能在真菌定殖过程中起重要作用。在DON处理的拟南芥中也能观察到这种现象,说明镰刀菌可能利用真菌毒素来抑制类似PCD的细胞凋亡,从而完成完整的生命周期[80]。UGT基因家族是植物中最大、功能最重要的基因家族之一,而糖基化反应被认为是植物次生代谢物最重要的修饰之一[81]。植物可以通过特定的UGT参与DON的解毒[82]。在小麦中,DON的解毒可以通过与GSH的结合或通过特定的UGTs催化的糖基化来进行,利用UGT将DON转化为无毒的DON-3-葡萄糖苷,而后者并不是毒力因子,植物利用此机制在被感染时使病原菌的毒力下降[83-85]。此外,在拟南芥[86]和小麦[87-88]中过表达UGT基因均可以使寄主获得DON抗性。UGT基因也参与大麦解毒过程,将有毒的ZEN转化为ZEN-14G、ZEN-16G、α-玉米赤霉烯醇-14-葡萄糖苷(α-ZOL-14G)、β-玉米赤霉烯醇-14-葡萄糖苷(β-ZOL-14G)和玉米赤霉烯酮-14, 16-二葡萄糖苷(ZAN-14, 16G)等无毒的化合物,降低毒素的致病力[89]。
3.2 转运蛋白将毒素运输到胞外或隔离多药物转运蛋白也是植物应对镰刀菌属毒素的策略之一。对于病原菌产生的毒素,寄主会采取液泡隔离降解或转运出胞外的方式以彻底去除毒素的毒害。植物ATP结合盒(ATP-binding cassette,ABC)转运蛋白已被证明在这一过程中发挥着核心作用[90]。ABC转运蛋白是一个膜转运蛋白大家族,定位于细胞膜、液泡膜、叶绿体、线粒体和过氧化物酶体中,参与细胞的选择性运输[91-92]。该类蛋白可将毒素与GSH结合形成的复合物转运至液泡中储存,以有效降低毒素对细胞的伤害[93]。
多药和有毒化合物排出(Multidrug and toxic com-pound extrusion,MATE)转运蛋白广泛存在于动物、植物和微生物中,能与一些潜在的毒性化合物结合,通过ATP/质子介导的过程运输到细胞外[94]。在拟南芥中发现的AtALF5是第一个被鉴定的植物MATE转运蛋白,主要在根中表达,参与拟南芥侧根的形成及根系毒素的解毒过程[95]。Wang等[96]发现拟南芥通过P4-ATPase介导的囊泡运输,将毒素从质膜转移到液泡中进行降解,以此提高植物对DON的抗性。
3.3 植物分泌次生代谢物抑制毒素合成相对于将已产生的毒素转化为无毒或低毒的化合物,植物通过分泌次生代谢物直接抑制毒素的生物合成是植物防御病原菌的另一重要策略[97]。植物分泌的次生代谢物可以分为3类:酚类、萜类和含氮化合物[98]。其中,酚类化合物一般指酚类、黄酮类和醌类。酚酸是一种存在于植物细胞壁的次生代谢物,具有抗过氧化活性[99]。Etzerodt等[100]研究表明,酚酸类物质能够抑制小麦中Tri的积累;鞣花酸能通过调控fum基因的表达,抑制轮枝镰刀菌中FB的生物合成[101];包括阿魏酸、香草酸和对羟基苯甲酸在内的酚酸已被证明可以降低镰刀菌中ZEN的含量[102]。现有研究表明,与植物苯丙烷代谢途径有关的次生代谢物与禾谷镰刀菌的防御和DON的产生相关,参与苯丙烷途径的次生代谢物超过一半属于黄酮类物质[103]。Perincherry等[104]研究表明,黄酮类化合物和酚酸类化合物均能够显著抑制层出镰刀菌分泌的FB和BEA。此外,苯并恶嗪类化合物(BXs)是一类广泛存在于禾本科植物中与防御相关的次生代谢物。已有报道指出,苯并噁嗪类化合物2-β吡喃葡糖苷-2, 4-二羟基-7-甲氧基-1, 4-苯并噁嗪-3-酮、α-生育酚及黄酮类化合物荭草苷和异荭草苷可能抑制小麦中DON的积累[105]。在禾谷镰刀菌侵染的玉米中,发现一种萜类植保素玉米凝集素,能够抑制禾谷镰刀菌的生长[106]。萜类植保素既是植物次生代谢物的组成部分,也在玉米先天性免疫中起重要作用。
一般来说,植物体内的ROS解毒是通过一系列具有氧化还原能力的酶和次生代谢物来实现的。植物分泌的次生代谢物,如黄酮类化合物的抗氧化作用机制之一便是通过抑制病原菌侵染过程中ROS的大量产生,以达到抵御病原菌的目的[98]。Paciolla等[53]证明了BEA和T-2毒素通过产生H2O2诱导细胞中的氧化应激,导致番茄原生质体活力降低。少量的ROS可以激活植物自身的防御反应,过多的ROS则会导致氧化应激损伤,对植物自身产生毒害[107]。尖孢镰刀菌侵染拟南芥根系会导致根部ROS的增加,毒素的产生会破坏植物在氧化胁迫期间被激活的抗坏血酸系统,以达到侵染目的[108]。
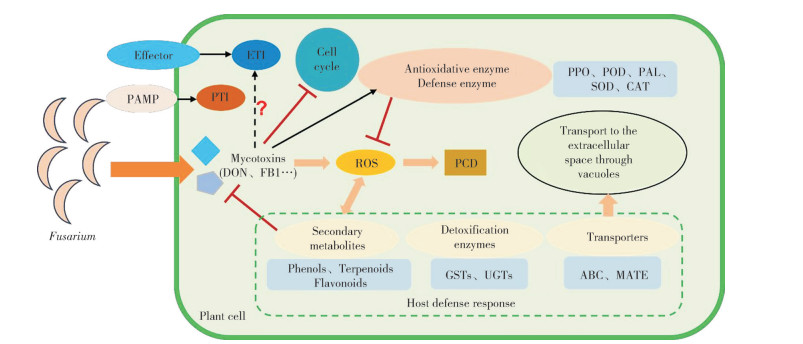
3.4 镰刀菌属毒素与植物互作模式综上,我们总结了镰刀菌属毒素与植物互作的模式图(图 1)。镰刀菌识别植物细胞后,分泌细胞壁降解酶,加速病原菌的侵染,这些物质可以作为PAMP,激发植物的病原体相关分子模式触发免疫(PAMP triggered immunity,PTI),也可以产生效应子激发植物的效应子触发免疫(Effector triggered immunity,ETI);此外,病原菌通过分泌致病毒素,抑制DNA复制,细胞循环受阻;也可以诱导植物抗氧化酶和防御酶的活性,激活自身的防御系统,大量的毒素可能会诱导ROS的产生,导致PCD。为应对真菌毒素的危害,植物通过产生解毒酶解毒、利用转运蛋白将毒素运输至胞外、分泌次生代谢物抑制毒素的产生,而这些次生代谢物也可以参与ROS的解毒作用。但毒素是否作为效应子诱导植物的ETI反应尚未见报道。
|
| 图 1 镰刀菌属毒素与植物互作的模式图 Fig. 1 Model of interaction between Fusarium mycotoxins and plants |
4 结语与展望
病原菌产生的真菌毒素对病原菌致病力具有十分重要的作用。目前,对于镰刀菌产生的真菌毒素的种类、生物合成途径、致病机理等都有了较为深入的了解。在病原菌与植物寄主的相互作用中,毒素一方面可加速病原菌对植物的侵染,增加其致病力;另一方面可能会使寄主在受病原菌侵染过程中识别病原菌,从而激活寄主的防御反应。由于镰刀菌属的真菌多存在于土壤中,是一种土传病原菌,防治起来极为困难。因此,在找到绿色、安全、有效的防控措施前,解析病原菌的致病机制将是今后的重点工作之一。系统理解毒素致病机理和与寄主植物间的互作机制,对利用病原菌毒素进行病害防治、植物抗病性鉴定和抗病突变体的筛选育种等具有重要意义。
为抵御病原菌,植物进化出强大的免疫系统,可分为两部分,一部分是细胞表面模式识别受体(Pattern recognition receptors,PRRs)触发的免疫反应,称为PTI,PTI可应对大多数病原菌的入侵。另一部分病原菌已经进化出逃避PTI的方法,通过向寄主植物细胞注入毒性因子(Virulence factors)或效应子(Effector molecules)来抑制植物的PTI,而一些效应子可被植物细胞内的核苷酸结合寡聚化结构域NOD样受体(Nucleotide binding domain and Leucine-rich Repeats,NLRs)识别后触发比PTI更强的免疫反应,称为ETI,此过程常常伴有PCD,又称HR。现有的病原菌和植物互作中的自身免疫反应的研究大都集中在由PRRs引起的PTI反应和由病原菌分泌的效应蛋白所引起的ETI反应,真菌毒素在植物免疫反应中的作用还未解析。因此,真菌毒素在病原菌侵染过程中引起的植物免疫反应可能是今后解析植物和病原菌互作机制的方向。
随着组学技术和小RNA研究的不断发展和完善,在今后的研究中,利用转录组、代谢组、基因组等多组学联合分析,挖掘病原菌的致病毒素,通过RNA干扰、基因敲除等技术研究毒素合成关键基因的致病功能和基因调控的生命过程,有助于了解真菌毒素在镰刀菌和寄主互作中的作用。Wang等[109]研究表明,在禾谷镰刀菌中,通过同时沉默编码DON生物合成关键调控基因FgSGE1(又称FGP1)、编码植物穿透结构形成关键转录因子的FgSTE12和编码必需磷酸酶的FgPP1,表达FgSGE1-STE12-PP1嵌合RNAi的宿主诱导基因沉默(Host-induced gene silencing,HIGS)转基因小麦植株在真菌侵染过程中增强了对赤霉病的抗性并抑制了DON的生物合成。这表明针对参与真菌初级和次级代谢的多基因HIGS是有效的。目前,已在镰刀菌中鉴定到了一些真菌毒素产生基因,这些基因的RNA干扰可能会抑制镰刀菌的生长和致病力,将为找到防控病原菌的有效靶点、制定病害综合防治策略提供科学依据。
| [1] |
TURNER N W, BRAMHMBHATT H, SZABO-VEZSE M, POMA A, COKER R, PILETSKY S A. Analytical methods for determination of mycotoxins: An update (2009-2014)[J]. Analytica Chimica Acta, 2015, 901: 12-33. DOI:10.1016/j.aca.2015.10.013 |
| [2] |
HAQUE M A, WANG Y, SHEN Z, LI X, SALEEMI M K, HE C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review[J]. Microbial Pathogenesis, 2020, 142: 104095. DOI:10.1016/j.micpath.2020.104095 |
| [3] |
RAI A, DAS M, TRIPATHI A. Occurrence and toxicity of a Fusarium mycotoxin, zearalenone[J]. Critical Reviews in Food Science and Nutrition, 2020, 60(16): 2710-2729. DOI:10.1080/10408398.2019.1655388 |
| [4] |
DEAN R, VAN KAN J A L, PRETORIUS Z A, HAMMOND-KOSACK K E, DI PIETRO A, SPANU P D, RUDD J J, DICKMAN M, KAHMANN R, ELLIS J, FOSTER G D. The top 10 fungal pathogens in molecular plant pathology[J]. Molecular Plant Pathology, 2012, 13(4): 414-430. DOI:10.1111/j.1364-3703.2011.00783.x |
| [5] |
李英, 杜春梅. 致病性尖孢镰刀菌毒力因子的研究进展[J]. 中国农学通报, 2021, 37(12): 92-97. DOI:10.11924/j.issn.1000-6850.casb2020-0389 LI Y, DU C M. Virulence factors of pathogenic Fusarium oxysporum: Research progress[J]. Chinese Agricultural Science Bulletin, 2021, 37(12): 92-97. DOI:10.11924/j.issn.1000-6850.casb2020-0389 |
| [6] |
李敏慧, 苑曼琳, 姜子德, 李华平. 香蕉枯萎病菌致病机理研究进展[J]. 果树学报, 2019, 36(6): 803-811. DOI:10.13925/j.cnki.gsxb.20180523 LI M H, YUAN M L, JIANG Z D, LI H P. Research progress in pathogenic mechanism of Fusarium oxysporum f. sp. cubense[J]. Journal of Fruit Science, 2019, 36(6): 803-811. DOI:10.13925/j.cnki.gsxb.20180523 |
| [7] |
PERINCHERRY L, LALAK-KANCZUGOWSKA J, STEPIEN L. Fusarium-produced mycotoxins in plant-pathogen interactions[J]. Toxins, 2019, 11(11): 664. DOI:10.3390/toxins11110664 |
| [8] |
KLÖTZEL M, LAUBER U, HUMPF H U. A new solid phase extraction clean-up method for the determination of 12 type A and B trichothecenes in cereals and cereal-based food by LC-MS/MS[J]. Molecular Nutrition & Food Research, 2006, 50(3): 261-269. DOI:10.1002/mnfr.200500234 |
| [9] |
KIMURA M, TOKAI T, TAKAHASHI-ANDO N, OHSATO S, FUJIMURA M. Molecular and genetic studies of Fusarium trichothecene biosynthesis: Pathways, genes, and evolution[J]. Bioscience, Biotechnology and Biochemistry, 2007, 71(9): 2105-2123. DOI:10.1271/bbb.70183 |
| [10] |
HOHN T M, DESJARDINS A E, MCCORMICK S P. The Tri4 gene of Fusarium sporotrichioides encodes a cytochrome P450 monooxygenase involved in trichothecene biosynthesis[J]. Molecular Genetics and Genomics, 1995, 248(1): 95-102. DOI:10.1007/bf02456618 |
| [11] |
TOKAI T, KOSHINO H, TAKAHASHI-ANDO N, SATO M, FUJIMURA M, KIMURA M. Fusarium Tri4 encodes a key multifunctional cytochrome P450 monooxygenase for four consecutive oxygenation steps in trichothecene biosynthesis[J]. Biochemical and Biophysical Research Communications, 2007, 353(2): 412-417. DOI:10.1016/j.bbrc.2006.12.033 |
| [12] |
ALEXANDER N J, PROCTOR R H, MCCORMICK S P. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium[J]. Toxin Reviews, 2009, 28(2/3): 198-215. DOI:10.1080/15569540903092142 |
| [13] |
FOROUD N A, BAINES D, GAGKAEVA T Y, THAKOR N, BADEA A, STEINER B, BüRSTMAYR M, BÜRSTMAYR H. Trichothecenes in cereal grains - an update[J]. Toxins (Basel), 2019, 11(11): 634. DOI:10.3390/toxins11110634 |
| [14] |
AHMED ADAM M A, TABANA Y M, MUSA K B, SANDAI D A. Effects of different mycotoxins on humans, cell genome and their involvement in cancer (Review)[J]. Oncology Reports, 2017, 37(3): 1321-1336. DOI:10.3892/or.2017.5424 |
| [15] |
ADHIKARI M, NEGI B, KAUSHIK N, ADHIKARI A, ALKHEDHAIRY A A, KAUSHIK N K, CHOI E H. T-2 mycotoxin: Toxicological effects and decontamination strategies[J]. Onco Targets and Therapy, 2017, 8(20): 33933-33952. DOI:10.18632/oncotarget.15422 |
| [16] |
DESJARDINS A E, MCCORMICK S P, APPELL M. Structure-activity relationships of trichothecene toxins in an Arabidopsis thaliana leaf assay[J]. Journal of Agricultural and Food Chemistry, 2007, 55(16): 6487-6492. DOI:10.1021/jf0709193 |
| [17] |
WAHIBAH N N, TSUTSUI T, TAMAOKI D, SATO K, NISHIUCHI T. Expression of barley glutathione S-transferase13 gene reduces accumulation of reactive oxygen species by trichothecenes and paraquat in Arabidopsis plants[J]. Plant Biotechnology Journal, 2018, 35(1): 71-79. DOI:10.5511/plantbiotechnology.18.0205a |
| [18] |
JANSEN C, VON WETTSTEIN D, SCHAFER W, KOGEL K H, FELK A, MAIER F J. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(46): 16892-16897. DOI:10.1073/pnas.0508467102 |
| [19] |
KOWALSKA K, HABROWSKA-GóRCZYŃSKA D E, PIASTOWSKA-CIESIELSKA A W. Zearalenone as an endocrine disr uptor in humans[J]. Environmental Toxicolog y and Pharmacology, 2016, 48: 141-149. DOI:10.1016/j.etap.2016.10.015 |
| [20] |
ZHANG G L, FENG Y L, SONG J L, ZHOU X S. Zearalenone: A mycotoxin with different toxic effect in domestic and laboratory animals' granulosa cells[J]. Frontiers in Genetics, 2018, 9: 667. DOI:10.3389/fgene.2018.00667 |
| [21] |
张卫娜, 贾谏, 陆晓宇, 陈中健, 孔谦, 陈庄. 镰刀菌属真菌毒素的研究进展[J]. 广东农业科学, 2013, 40(15): 130-133. DOI:10.16768/j.issn.1004-874X.2013.15.031 ZHANG W N, JIA J, LU X Y, CHEN Z J, KONG Q, CHEN Z. Research advance on Fusarium mycotoxins[J]. Guangdong Agricultural Sciences, 2013, 40(15): 130-133. DOI:10.16768/j.issn.1004-874X.2013.15.031 |
| [22] |
ZHU Y, WANG H, WANG J, HAN S, ZHANG Y, MA M, ZHU Q, ZHANG K, YIN H. Zearalenone induces apoptosis and cytoprotective autophagy in chicken granulosa cells by PI3K-AKT-mTOR and MAPK signaling pathways[J]. Toxins (Basel), 2021, 13(3): 199. DOI:10.3390/toxins13030199 |
| [23] |
BORZEKOWSKI A, DREWITZ T, KELLER J, PFEIFER D, KUNTE H J, KOCH M, ROHN S, MAUL R. Biosynthesis and characterization of zearalenone-14-sulfate, zearalenone-14-glucoside and zearalenone-16-glucoside using common fungal strains[J]. Toxins (Basel), 2018, 10(3): 104. DOI:10.3390/toxins10030104 |
| [24] |
STĘPIEŃ L, KOCZYK G, WAŚKIEWICZ A. FUM cluster divergence in fumonisins-producing Fusarium species[J]. Fungal Biology, 2011, 115(2): 112-123. DOI:10.1016/j.funbio.2010.10.011 |
| [25] |
BROWN D W, CHEUNG F, PROCTOR R H, BUTCHKO R A, ZHENG L, LEE Y, UTTERBACK T, SMITH S, FELDBLYUM T, GLENN A E, PLATTNER R D, KENDRA D F, TOWN C D, WHITELAW C A. Comparative analysis of 87, 000 expressed sequence tags from the fumonisin-producing fungus Fusarium verticillioides[J]. Fungal Genetics and Biology, 2005, 42(10): 848-861. DOI:10.1016/j.fgb.2005.06.001 |
| [26] |
STOCKMANN-JUVALA H, SAVOLAINEN K. A review of the toxic effects and mechanisms of action of fumonisin B1[J]. Human & Experimental Toxicology, 2008, 27(11): 799-809. DOI:10.1177/0960327108099525 |
| [27] |
秦小雅, 尹跃, 梁晓婕, 赵建华, 安巍, 曹有龙. 伏马菌素FB1引起枸杞生长抑制及衰老的初步分析[J/OL]. 分子植物育种, 2021: 1-12. QING X Y, YING Y, LIANG X J, ZHAO J H, AN W, CAO Y L. A preliminary analyzation of growth inhibition and aging caused by 19 fumonisin FB1 in Lycium bararum L. [J/OL]. Molecular Plant Breeding, 2021: 1-12. |
| [28] |
LI T, SU X, QU H, DUAN X, JIANG Y. Biosynthesis, regulation, and biological significance of fumonisins in fungi: Current status and prospects[J]. Critical Reviews in Microbiology, 2022, 48(4): 450-462. DOI:10.1080/1040841x.2021.1979465 |
| [29] |
XING F, LI Z, SUN A, XING D. Reactive oxygen species promote chloroplast dysfunction and salicylic acid accumulation in fumonisin B1-induced cell death[J]. Febs Letters, 2013, 587(14): 2164-2172. DOI:10.1016/j.febslet.2013.05.034 |
| [30] |
IQBAL N, CZÉKUS Z, POÓR P, ÖRDÖG A. Plant defence mechanisms against mycotoxin fumonisin B1[J]. Chemico-Biological Interactions, 2021, 343: 109494. DOI:10.1016/j.cbi.2021.109494 |
| [31] |
郭志青, 张霞, 刁立功, 许曼琳, 于静, 李莹, 何康, 宋新颖, 王维婷, 迟玉成. 镰刀菌及其伏马毒素的危害和防控[J]. 山东农业科学, 2022, 54(1): 157-164. DOI:10.14083/j.issn.1001-4942.2022.01.024 GUO Z Q, ZHANG X, DIAO L G, XU M L, YU J, LI Y, HE K, SONG X Y, WANG W T, CHI Y C. Hazards and control of Fusarium spp. and its metabolites fumonisinse[J]. Shandong Agricultural Sciences, 2022, 54(1): 157-164. DOI:10.14083/j.issn.1001-4942.2022.01.024 |
| [32] |
NIEHAUS E M, KIM H K, MÜNSTERKÖTTER M, JANEVSKA S, ARNDT B, KALININA S A, HOUTERMAN P M, AHN I P, ALBERTI I, TONTI S, KIM D W, SIEBER C M K, HUMPF H U, YUN S H, GüLDENER U, TUDZYNSKI B. Comparative genomics of geographically distant Fusarium fujikuroi isolates revealed two distinct pathotypes correlating with secondary metabolite profiles[J]. PLoS Pathogens, 2017, 13(10): e1006670. DOI:10.1371/journal.ppat.1006670 |
| [33] |
XU F, HUANG L, WANG J, MA C, TAN Y, WANG F, FAN Y, LUO M. Sphingolipid synthesis inhibitor fumonisin B1 causes verticillium wilt in cotton[J]. Journal of Integrative Plant Biology, 2022, 64(4): 836-842. DOI:10.1111/jipb.13241 |
| [34] |
XIE L, WU Y, WANG Y, JIANG Y, YANG B, DUAN X, LI T. Fumonisin B1 induced aggressiveness and infection mechanism of Fusarium proliferatum on banana fruit[J]. Environmental Pollution, 2021, 288: 117793. DOI:10.1016/j.envpol.2021.117793 |
| [35] |
LÓPEZ-DÍAZ C, RAHJOO V, SULYOK M, GHIONNA V, MARTÍN-VICENTE A, CAPILLA J, DI PIETRO A, LÓPEZ-BERGES M S. Fusaric acid contributes to virulence of Fusarium oxysporum on plant and mammalian hosts[J]. Molecular Plant Pathology, 2018, 19(2): 440-453. DOI:10.1111/mpp.12536 |
| [36] |
SINGH V K, UPADHYAY R S. Fusaric acid induced cell death and changes in oxidative metabolism of Solanum lycopersicum L[J]. Botanical Studies, 2014, 55(1): 66. DOI:10.1186/s40529-014-0066-2 |
| [37] |
BROWN D W, LEE S-H, KIM L-H, RYU J-G, LEE S, SEO Y, KIM Y H, BUSMAN M, YUN S-H, PROCTOR R H, LEE T. Identification of a 12-gene fusaric acid biosynthetic gene cluster in Fusarium species through comparative and functional genomics[J]. Molecular Plant-Microbe Interactions, 2015, 28(3): 319-332. DOI:10.1094/mpmi-09-14-0264-r |
| [38] |
LIU S, LI J, ZHANG Y, LIU N, VILJOEN A, MOSTERT D, ZUO C, HU C, BI F, GAO H, SHENG O, DENG G, YANG Q, DONG T, DOU T, YI G, MA L J, LI C. Fusaric acid instigates the invasion of banana by Fusarium oxysporum f. sp. cubense TR4[J]. New Phytologist, 2020, 225(2): 913-929. DOI:10.1111/nph.16193 |
| [39] |
COLE R J, KIRKSEY J W, CUTLER H G, DOUPNIK B L, PECKHAM J C. Toxin from Fusarium moniliforme: Effects on plants and animals[J]. Science, 1973, 179(4080): 1324-1326. DOI:10.1126/science.179.4080.1324 |
| [40] |
SHARMA D, ASRANI R K, LEDOUX D R, ROTTINGHAUS G E, GUPTA V K. Toxic interaction between fumonisin B1 and moniliformin for cardiac lesions in Japanese quail[J]. Avian Diseases, 2012, 56(3): 545-554. DOI:10.1637/10036-121111-Reg.1 |
| [41] |
ESCRIVA L, FONT G, MANYES L. In vivo toxicity studies of fusarium mycotoxins in the last decade: A review[J]. Food and Chemical Toxicology, 2015, 78: 185-206. DOI:10.1016/j.fct.2015.02.005 |
| [42] |
FRAEYMAN S, CROUBELS S, DEVREESE M, ANTONISSEN G. Emerging Fusarium and Alternaria mycotoxins: Occurrence, toxicity and toxicokinetics[J]. Toxins, 2017, 9(7): 228. DOI:10.3390/toxins9070228 |
| [43] |
PELTONEN K, JESTOI M, ERIKSEN G S. Health effects of moniliformin a poorly understood Fusarium mycotoxin[J]. World Mycotoxin Journal, 2010, 3(4): 403-414. DOI:10.3920/wmj2010.1232 |
| [44] |
田雪亮, 郎剑锋, 周建, 陆宁海, 陈锡岭. 串珠镰刀菌粗毒素对玉米根系细胞膜的影响[J]. 广东农业科学, 2012, 39(6): 87-88, 97. DOI:10.16768/j.issn.1004-874X.2012.06.048 TIAN X L, LANG J F, ZHOU J, LU N H, CHEN X L. Effect of crude toxin produced by Fusarium moniliforme on cell membrane of maize root[J]. Guangdong Agricultural Sciences, 2012, 39(6): 87-88, 97. DOI:10.16768/j.issn.1004-874X.2012.06.048 |
| [45] |
SORENSEN J L, PHIPPS R K, NIELSEN K F, SCHROERS H-J, FRANK J, THRANE U. Analysis of Fusarium avenaceum metabolites produced during wet apple core rot[J]. Journal of Agricultural and Food Chemistry, 2009, 57(4): 1632-1639. DOI:10.1021/jf802926u |
| [46] |
张晓伟, 张栋. 镰孢菌属真菌次生代谢产物的研究进展[J]. 植物生理学报, 2013, 49(3): 201-216. DOI:10.13592/j.cnki.ppj.2013.03.001 ZHANG X W, ZHANG D. Recent advances of secondary metabolites in genus Fusarium[J]. Plant Physiology Journal, 2013, 49(3): 201-216. DOI:10.13592/j.cnki.ppj.2013.03.001 |
| [47] |
CALONI F, FOSSATI P, ANADON A, BERTERO A. Beauvericin: The beauty and the beast[J]. Environmental Toxicology and Pharmacology, 2020, 75: 103349. DOI:10.1016/j.etap.2020.103349 |
| [48] |
WU Q H, PATOCKA J, KUCA K. Beauvericin, a Fusarium mycotoxin: Anticancer activity, mechanisms, and human exposure risk assessment[J]. Mini-Reviews in Medicinal Chemistry, 2019, 19(3): 206-214. DOI:10.2174/1389557518666180928161808 |
| [49] |
NIEHAUS E M, STUDT L, VON BARGEN K W, KUMMER W, HUMPF H U, REUTER G, TUDZYNSKI B. Sound of silence: The beauvericin cluster in Fusarium fujikuroi is controlled by cluster-specific and global regulators mediated by H3K27 modification[J]. Environmental Microbiology, 2016, 18(11): 4282-4302. DOI:10.1111/1462-2920.13576 |
| [50] |
FUMERO M V, VILLANI A, SUSCA A, HAIDUKOWSKI M, CIMMARUSTI M T, TOOMAJIAN C, LESLIE J F, CHULZE S N, MORETTI A. Fumonisin and beauvericin chemotypes and genotypes of the sister species Fusarium subglutinans and Fusarium temperatum[J]. Applied and Environmental Microbiology, 2020, 86(13): e00133-20. DOI:10.1128/aem.00133-20 |
| [51] |
MALLEBRERA B, PROSPERINI A, FONT G, RUIZ M J. In vitro mechanisms of beauvericin toxicity: A review[J]. Food and Chemical Toxicology, 2018, 111: 537-545. DOI:10.1016/j.fct.2017.11.019 |
| [52] |
WU Q, PATOCKA J, NEPOVIMOVA E, KUCA K. A review on the synthesis and bioactivity aspects of beauvericin, a Fusarium mycotoxin[J]. Frontiers in Pharmacology, 2018, 9: 1338. DOI:10.3389/fphar.2018.01338 |
| [53] |
PACIOLLA C, DIPIERRO N, MULE G, LOGRIECO A, DIPIERRO S. The mycotoxins beauvericin and T-2 induce cell death and alteration to the ascorbate metabolism in tomato protoplasts[J]. Physiological and Molecular Plant Pathology, 2004, 65(1): 49-56. DOI:10.1016/j.pmpp.2004.07.006 |
| [54] |
李春雨, 陈石, 左存武, 邝瑞彬, 易干军. 香蕉枯萎病菌新毒素——白僵菌素的鉴定[J]. 园艺学报, 2011, 38(11): 2092-2098. DOI:10.16420/j.issn.0513-353x.2011.11.002 LI C Y, CHEN S, ZUO C W, KUANG R B, YI G J. Identification of beauvericin, a novel mycotoxin from Fusarium oxysporum f. sp. cubense[J]. Acta Horticulturae Sinica, 2011, 38(11): 2092-2098. DOI:10.16420/j.issn.0513-353x.2011.11.002 |
| [55] |
DORNETSHUBER-FLEISS R, HEILOS D, MOHR T, RICHTER L, SUESSMUTH R D, ZLESAK M, NOVICKY A, HEFFETER P, LEMMENS-GRUBER R, BERGER W. The naturally born fusariotoxin enniatin B and sorafenib exert synergistic activity against cervical cancer in vitro and in vivo[J]. Biochemical Pharmacology, 2015, 93(3): 318-331. DOI:10.1016/j.bcp.2014.12.013 |
| [56] |
BERTERO A, MORETTI A, SPICER L J, CALONI F. Fusarium molds and mycotoxins: Potential species-specific effects[J]. Toxins, 2018, 10(6): 244. DOI:10.3390/toxins10060244 |
| [57] |
BERTERO A, FOSSATI P, TEDESCO D E A, CALONI F. Beauvericin and enniatins: In vitro intestinal effects[J]. Toxins, 2020, 12(11): 686. DOI:10.3390/toxins12110686 |
| [58] |
GNONLONFOUN E, FOTIN G, RISLER A, ELFASSY A, SCHWEBEL S, SCHMITT M, BORGES F, MANGAVEL C, REVOL-JUNELLES A-M, FICK M, FRAMBOISIER X, RONDAGS E. Inhibition of the growth of Fusarium tricinctum and reduction of its enniatin production by Erwinia gerundensis isolated from barley kernels[J]. Journal of the American Society of Brewing Chemists, 2023, 81(2): 340-350. DOI:10.1080/03610470.2022.2041970 |
| [59] |
张嘉城, 方香玲, 南志标. 植物病原镰刀菌产生的毒素种类及其危害[J]. 草业科学, 2021, 38(8): 1513-1524. DOI:10.11829/j.issn.1001-0629.2020-0716 ZHANG J C, FANG X L, NAN Z B. Ty pes a nd ef fects of toxins produced by plant pathogenic fungi Fusarium[J]. Pratacultural Science, 2021, 38(8): 1513-1524. DOI:10.11829/j.issn.1001-0629.2020-0716 |
| [60] |
ERANTHODI A, SCHNEIDERMAN D, HARRIS L J, WITTE T E, SPROULE A, HERMANS A, OVERY D P, CHATTERTON S, LIU J, LI T, FUNDORA D G, ZHAO W, FOROUD N A. Enniatin production influences Fusarium avenaceum virulence on potato tubers, but not on durum wheat or peas[J]. Pathogens, 2020, 9(2): 75. DOI:10.3390/pathogens9020075 |
| [61] |
EDERLI L, BECCARI G, TINI F, BERGAMINI I, BELLEZZA I, ROMANI R, COVARELLI L. Enniatin B and deoxynivalenol activity on bread wheat and on Fusarium species development[J]. Toxins, 2021, 13(10): 728. DOI:10.3390/toxins13100728 |
| [62] |
MICHIELSE C B, REP M. Pathogen profile update: Fusarium oxysporum[J]. Molecular Plant Pathology, 2009, 10(3): 311-324. DOI:10.1111/j.1364-3703.2009.00538.x |
| [63] |
KAZAN K, GARDINER D M. Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: Recent progress and future prospects[J]. Molecular Plant Pathology, 2018, 19(7): 1547-1562. DOI:10.1111/mpp.12639 |
| [64] |
吴元立, 杨乔松, 李春雨, 黄秉智, 董涛, 盛鸥, 毕方铖, 邓贵明, 胡春华, 高慧君, 窦同心, 何维弟, 刘思文, 易干军. 香蕉-尖孢镰刀菌互作机理及抗病育种研究进展[J]. 广东农业科学, 2020, 47(11): 32-41. DOI:10.16768/j.issn.1004-874X.2020.11.004 WU Y L, YANG Q S, LI C Y, HUANG B Z, DONG T, SHENG O, BI F C, DENG G M, HU C H, GAO H J, DOU T X, HE W D, LIU S W, YI G J. Research progress in the mechanisms of banana-Fusarium oxysporum f. sp. cubense interaction and genetic improvement for resistance to Fusarium wilt[J]. Guangdong Agricultural Sciences, 2020, 47(11): 32-41. DOI:10.16768/j.issn.1004-874X.2020.11.004 |
| [65] |
LI C, YANG J, LI W, SUN J, PENG M. Direct root penetration and rhizome vascular colonization by Fusarium oxysporum f. sp cubense are the key steps in the successful infection of Brazil Cavendish[J]. Plant Disease, 2017, 101(12): 2073-2078. DOI:10.1094/pdis-04-17-0467-re |
| [66] |
PERINCHERRY L, URBANIAK M, PAWLOWICZ I, KOTOWSKA K, WASKIEWICZ A, STEPIEN L. Dynamics of Fusarium mycotoxins and lytic enzymes during pea plants' infection[J]. International Journal of Molecular Sciences, 2021, 22(18): 9888. DOI:10.3390/ijms22189888 |
| [67] |
KUBICEK C P, STARR T L, GLASS N L. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi[J]. Annual Review of Phytopathology, 2014, 52: 427-451. DOI:10.1146/annurev-phyto-102313-045831 |
| [68] |
王珊珊, 乜兰春, 李潘, 王政. 植物病原真菌毒素的分类、致病机制及应用前景[J]. 江苏农业科学, 2019, 47(3): 94-97. DOI:10.15889/j.issn.1002-1302.2019.03.022 WANG S S, NIE C L, LI P, WANG Z. Classification, pathogenic mechanisms, and application prospects of plant pathogenic mycotoxins[J]. Jiangsu Agricultural Sciences, 2019, 47(3): 94-97. DOI:10.15889/j.issn.1002-1302.2019.03.022 |
| [69] |
陈慧杰, 赵爽, 张凯凯, 邹忠幸, 倪嘉琪, 姜晓帆, 陈发棣, 房伟民. 菊花枯萎病病原菌的分离和鉴定及其粗毒素对切花菊'神马'幼苗生长的影响[J]. 南京农业大学学报, 2018, 41(4): 662-669. DOI:10.7685/jnau.201708009 CHEN H J, ZHANG S, ZHANG K K, ZOU Z X, NI J Q, JIANG X F, CHEN F D, FANG W M. Isolation and identification of Fusarium oxysporum from chrysanthemum and the effects of crude toxin on growth of cut-chrysanthemum 'Jimba' seedling[J]. Journal of Nanjing Agricultural University, 2018, 41(4): 662-669. DOI:10.7685/jnau.201708009 |
| [70] |
WANG R, HUANG J, LIANG A, WANG Y, MUR L A J, WANG M, GUO S. Zinc and copper enhance cucumber tolerance to fusaric acid by mediating its distribution and toxicity and modifying the antioxidant system[J]. International Journal of Molecular Sciences, 2020, 21(9). DOI:10.3390/ijms21093370 |
| [71] |
WU H S, YIN X M, LIU D Y, LING N, BAO W, YING R R, ZHU Y Y, GUO S W, SHEN Q R. Effect of fungal fusaric acid on the root and leaf physiology of watermelon (Citrullus lanatus) seedlings[J]. Plant and Soil, 2008, 308(2): 255-266. DOI:10.1007/s11104-008-9627-z |
| [72] |
龙国辉, 武鹏雨, 付嘉智, 鹿宏丽, 张锐. 过氧化物酶调控木质素合成研究进展[J]. 现代农业科技, 2021(23): 47-49, 54. DOI:10.3969/j.issn.1007-5739.2021.23.019 LONG G H, WU P Y, FU J Z, LU H L, ZHANG R. Research progress on regulation of peroxidase on lignin synthesis[J]. Modern Agricultural Science and Technology, 2021(23): 47-49, 54. DOI:10.3969/j.issn.1007-5739.2021.23.019 |
| [73] |
潘亚清, 史淑芝. 植物的诱导抗病性研究进展[J]. 中国农学通报, 2005, 21(8): 366-369. DOI:10.3969/j.issn.1000-6850.2005.08.104 PAN Y Q, SHI S Z. Advances in study of plant induced disease resistance[J]. Chinese Agricultural Science Bulletin, 2005, 21(8): 366-369. DOI:10.3969/j.issn.1000-6850.2005.08.104 |
| [74] |
邓路长, 崔丽娜, 杨麟, 陈洁, 何文铸, 李晓, 唐海涛, 张彪, 康继伟, 杨俊品, 谭君. 玉米苯丙氨酸解氨酶家族基因的鉴定与纹枯病的抗病分析[J]. 分子植物育种, 2019, 17(3): 891-897. DOI:10.13271/j.mpb.017.000891 DENG L C, CUI L N, YANG L, CHEN J, HE W Z, LI X, TANG H T, ZHANG B, KANG J W, YANG J P, TAN J. Identification of gene family of phenylalanine ammonia-lyase and analysis of resistance to maize sheath blight in corn[J]. Molecular Plant Breeding, 2019, 17(3): 891-897. DOI:10.13271/j.mpb.017.000891 |
| [75] |
赵潇璨, 徐永清, 贺付蒙, 孙美丽, 袁强, 王雪, 孔德兴, 刘丹, 冯艳忠, 陈赫书, 田明, 刘娣, 李凤兰. 低浓度呕吐毒素作为激发子对马铃薯抗干腐病的诱导及其作用机制[J]. 作物学报, 2020, 46(11): 1801-1809. DOI:10.3724/sp.j.1006.2020.04049 ZHAO X C, XU Y Q, HE F M, SUN M L, YUAN Q, WANG X, KONG D X, LIU D, FENG Y Z, CHEN H S, TIAN M, LIU D, LI F L. Low concentration of vomitoxin as elicitor induced resistance of dry rot disease of potato and its mechanism[J]. Acta Agronomica Sinica, 2020, 46(11): 1801-1809. DOI:10.3724/sp.j.1006.2020.04049 |
| [76] |
LUO K, GUO J, HE D, LI G, OUELLET T. Deoxynivalenol accumulation and detoxification in cereals and its potential role in wheat-Fusarium graminearum interactions[J]. Abiotech, 2023, 4(2): 155-171. DOI:10.1007/s42994-023-00096-7 |
| [77] |
BERTHILLER F, CREWS C, DALL'ASTA C, DE SAEGER S, HAESAERT G, KARLOVSKY P, OSWALD I P, SEEFELDER W, SPEIJERS G, STROKA J. Masked mycotoxins: A review[J]. Molecular Nutrition & Food Research, 2013, 57(1): 165-186. DOI:10.1002/mnfr.201100764 |
| [78] |
WANG H, SUN S, GE W, ZHAO L, HOU B, WANG K, LYU Z, CHEN L, XU S, GUO J, LI M, SU P, LI X, WANG G, BO C, FANG X, ZHUANG W, CHENG X, WU J, DONG L, CHEN W, LI W, XIAO G, ZHAO J, HAO Y, XU Y, GAO Y, LIU W, LIU Y, YIN H, LI J, LI X, ZHAO Y, WANG X, NI F, MA X, LI A, XU S S, BAI G, NEVO E, GAO C, OHM H, KONG L. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat[J]. Science, 2020, 368: 5435. DOI:10.1126/science.aba5435 |
| [79] |
CHETOUHI C, BONHOMME L, LASSERRE-ZUBER P, CAMBON F, PELLETIER S, RENOU J P, LANGIN T. Transcriptome dynamics of a susceptible wheat upon Fusarium head blight reveals that molecular responses to Fusarium graminearum infection fit over the grain development processes[J]. Functional & Integrative Genomics, 2016, 16(2): 183-201. DOI:10.1007/s10142-016-0476-1 |
| [80] |
DIAMOND M, REAPE T J, ROCHA O, DOYLE S M, KACPRZYK J, DOOHAN F M, MCCABE P F. The Fusarium mycotoxin deoxynivalenol can inhibit plant apoptosis-like programmed cell death[J]. PLoS One, 2013, 8(7): e69542. DOI:10.1371/journal.pone.0069542 |
| [81] |
HUANG J, PANG C, FAN S, SONG M, YU J, WEI H, MA Q, LI L, ZHANG C, YU S. Genome-wide analysis of the family 1 glycosyltransferases in cotton[J]. Molecular Genetics and Genomics, 2015, 290(5): 1805-1818. DOI:10.1007/s00438-015-1040-8 |
| [82] |
MANDALA G, CEOLONI C, BUSATO I, FAVARON F, TUNDO S. Transgene pyramiding in wheat: Combination of deoxynivalenol detoxification with inhibition of cell wall degrading enzymes to contrast Fusarium head blight and crown rot[J]. Plant Science, 2021, 313: 111059. DOI:10.1016/j.plantsci.2021.111059 |
| [83] |
KLUGER B, BUESCHL C, LEMMENS M, MICHLMAYR H, MALACHOVA A, KOUTNIK A, MALOKU I, BERTHILLER F, ADAM G, KRSKA R, SCHUHMACHER R. Biotransformation of the mycotoxin deoxynivalenol in Fusarium resistant and susceptible near isogenic wheat lines[J]. PLoS One, 2015, 10(3): e0119656. DOI:10.1371/journal.pone.0119656 |
| [84] |
HE Y, AHMAD D, ZHANG X, ZHANG Y, WU L, JIANG P, MA H X. Genome-wide analysis of family-1 UDP glycosyltransferases (UGT) and identification of UGT genes for FHB resistance in wheat (Triticum aestivum L.)[J]. BMC Plant Biology, 2018, 18(1): 67. DOI:10.1186/s12870-018-1286-5 |
| [85] |
KHAIRULLINA A, MICIC N, JORGENSEN H J L, BJARNHOLT N, BULOW L, COLLINGE D B, JENSEN B. Biocontrol effect of Clonostachys rosea on Fusarium graminearum infection and mycotoxin detoxification in oat (Avena sativa)[J]. Plants (Basel), 2023, 12(3): 500. DOI:10.3390/plants12030500 |
| [86] |
POPPENBERGER B, BERTHILLER F, LUCYSHYN D, SIEBERER T, SCHUHMACHER R, KRSKA R, KUCHLER K, GLöSSL J, LUSCHNIG C, ADAM G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana[J]. Journal of Biological Chemistry, 2003, 278(48): 47905-47914. DOI:10.1074/jbc.M307552200 |
| [87] |
ZHAO L, MA X, SU P, GE W, WU H, GUO X, LI A, WANG H, KONG L. Cloning and characterization of a specific UDP-glycosyltransferase gene induced by DON and Fusarium graminearum[J]. Plant Cell Reports, 2018, 37(4): 641-652. DOI:10.1007/s00299-018-2257-x |
| [88] |
LI X, SHIN S, HEINEN S, DILL-MACKY R, BERTHILLER F, NERSESIAN N, CLEMENTE T, MCCORMICK S, MUEHLBAUER G J. Transgenic wheat expressing a barley UDP-glucosyltransferase detoxifies deoxynivalenol and provides high levels of resistance to Fusarium graminearum[J]. Molecular Plant-Microbe Interactions, 2015, 28(11): 1237-1246. DOI:10.1094/mpmi-03-15-0062-r |
| [89] |
MICHLMAYR H, VARGA E, LUPI F, MALACHOVA A, HAMETNER C, BERTHILLER F, ADAM G. Synthesis of mono- and di-glucosides of zearalenone and α-/β-zearalenol by recombinant barley glucosyltransferase HvUGT14077[J]. Toxins, 2017, 9(2): 58. DOI:10.3390/toxins9020058 |
| [90] |
HWANG J U, SONG W Y, HONG D, KO D, YAMAOKA Y, JANG S, YIM S, LEE E, KHARE D, KIM K, PALMGREN M, YOON H S, MARTINOIA E, LEE Y. Plant ABC transporters enable many unique aspects of a terrestrial plant's lifestyle[J]. Molecular Plant, 2016, 9(3): 338-355. DOI:10.1016/j.molp.2016.02.003 |
| [91] |
DEMISSIE Z A, TARNOWYCZ M, ADAL A M, SARKER L S, MAHMOUD S S. A lavender ABC transporter confers resistance to monoterpene toxicity in yeast[J]. Planta, 2019, 249(1): 139-144. DOI:10.1007/s00425-018-3064-x |
| [92] |
DAHUJA A, KUMAR R R, SAKHARE A, WATTS A, SINGH B, GOSWAMI S, SACHDEV A, PRAVEEN S. Role of ATP-binding cassette transporters in maintaining plant homeostasis under abiotic and biotic stresses[J]. Physiologia Plantarum, 2021, 171(4): 785-801. DOI:10.1111/ppl.13302 |
| [93] |
廖景燕. 真菌源ABCC转运蛋白对提高植物真菌毒素耐受性的作用研究[D]. 重庆: 西南大学, 2018. LIAO J Y. Assay of fungal ABCC-type transporters on enhancing tolerance of plant to several mycotoxins[D]. Chongqing: Southwest University, 2018. |
| [94] |
朱楚霞, 廖伟民, 刘建萍, 胡朝阳, 刘世强, 周勇. 黄瓜MATE家族基因鉴定及其响应根结线虫和白粉病菌侵染表达分析[J]. 江西农业大学学报, 2022, 44(2): 329-342. DOI:10.13836/j.jjau.2022034 ZHU C X, LIAO W M, LIU J P, HU C Y, LIU S Q, ZHOU Y. Genome-wide identification of the MATE family genes in cucumber and their responses to infection of root knot nematode and powdery mildew[J]. Acta Agriculturae Universitatis Jiangxiensis, 2022, 44(2): 329-342. DOI:10.13836/j.jjau.2022034 |
| [95] |
DIENER A C, GAXIOLA R A, FINK G R. Arabidopsis ALF5, a multidrug efflux transporter gene family member, confers resistance to toxins[J]. Plant Cell, 2001, 13(7): 1625-1638. DOI:10.1105/tpc.010035 |
| [96] |
WANG F L, LI X B, LI Y J, HAN J, CHEN Y, ZENG J Y, SU M, ZHUO J X, REN H, LIU H R, HOU L, FAN Y H, YAN X Y, SONG S Q, ZHAO J, JIN D, ZHANG M, PEI Y. Arabidopsis P4 ATPase-mediated cell detoxification confers resistance to Fusarium graminearum and Verticillium dahliae[J]. Nature Communications, 2021, 12(1): 6424. DOI:10.1038/s41467-021-26727-5 |
| [97] |
ATANASOVA-PENICHON V, BARREAU C, RICHARD-FORGET F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation[J]. Frontiers in Microbiology, 2016, 7: 566. DOI:10.3389/fmicb.2016.00566 |
| [98] |
ZAYNAB M, FATIMA M, ABBAS S, SHARIF Y, UMAIR M, ZAFAR M H, BAHADAR K. Role of secondary metabolites in plant defense against pathogens[J]. Microbial Pathogenesis, 2018, 124: 198-202. DOI:10.1016/j.micpath.2018.08.034 |
| [99] |
SHI M, HUANG F, DENG C, WANG Y, KAI G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza[J]. Critical Reviews in Food Science and Nutrition, 2018, 59(6): 953-964. DOI:10.1080/10408398.2018.1474170 |
| [100] |
ETZERODT T, MAEDA K, NAKAJIMA Y, LAURSEN B, FOMSGAARD I S, KIMURA M. 2, 4-dihydroxy-7-methoxy-2H-1, 4-benzoxazin-3(4H)-one (DIMBOA) inhibits trichothecene production by Fusarium graminearum through suppression of Tri6 expression[J]. International Journal of Food Microbiology, 2015, 214: 123-128. DOI:10.1016/j.ijfoodmicro.2015.07.014 |
| [101] |
FERRIGO D, BHARTI S, MONDIN M, RAIOLA A. Effect of naturally occurring compounds on fumonisin production and fum gene expression in Fusarium verticillioides[J]. Agronomy, 2021, 11(6): 1060. DOI:10.3390/agronomy11061060 |
| [102] |
SCHÖNEBERG T, KIBLER K, SULYOK M, MUSA T, BUCHELI T D, MASCHER F, BERTOSSA M, VOEGELE R T, VOGELGSANG S. Can plant phenolic compounds reduce Fusarium growth and mycotoxin production in cereals?[J]. Food Additives and Contaminants, 2018, 35(12): 2455-2470. DOI:10.1080/19440049.2018.1538570 |
| [103] |
GAUTHIER L, ATANASOVA-PENICHON V, CHÉREAU S, RICHARD-FORGET F. Metabolomics to decipher the chemical defense of cereals against Fusarium graminearum and deoxynivalenol accumulation[J]. International Journal of Molecular Sciences, 2015, 16(10): 24839-24872. DOI:10.3390/ijms161024839 |
| [104] |
PERINCHERRY L, WITASZAK N, URBANIAK M, WAŚKIEWICZ A, STĘPIEŃ L. Effects of secondary metabolites from pea on Fusarium growth and mycotoxin biosynthesis[J]. Journal of Fungi, 2021, 7(12): 1004. DOI:10.3390/jof7121004 |
| [105] |
ETZERODT T, GISLUM R, LAURSEN B B, HEINRICHSON K, GREGERSEN P L, JORGENSEN L N, FOMSGAARD I S. Correlation of deoxynivalenol accumulation in Fusarium-infected winter and spring wheat cultivars with secondary metabolites at different growth stages[J]. Journal of Agricultural and Food Chemistry, 2016, 64(22): 4545-4555. DOI:10.1021/acs.jafc.6b01162 |
| [106] |
HUFFAKER A, KAPLAN F, VAUGHAN M M, DAFOE N J, NI X, ROCCA J R, ALBORN H T, TEAL P E, SCHMELZ E A. Novel acidic sesquiterpenoids constitute a dominant class of pathogen-induced phytoalexins in maize[J]. Plant Physiology, 2011, 156(4): 2082-2097. DOI:10.1104/pp.111.179457 |
| [107] |
SEGAL L M, WILSON R A. Reactive oxygen species metabolism and plant-fungal interactions[J]. Fungal Genetics and Biology, 2018, 110: 1-9. DOI:10.1016/j.fgb.2017.12.003 |
| [108] |
LEHMANN S, SERRANO M, L'HARIDON F, TJAMOS S E, METRAUX J P. Reactive oxygen species and plant resistance to fungal pathogens[J]. Phytochemistry, 2015, 112: 54-62. DOI:10.1016/j.phytochem.2014.08.027 |
| [109] |
WANG M, WU L, MEI Y, ZHAO Y, MA Z, ZHANG X, CHEN Y. Host-induced gene silencing of multiple genes of Fusarium graminearum enhances resistance to Fusarium head blight in wheat[J]. Plant Biotechnology Journal, 2020, 18(12): 2373-2375. DOI:10.1111/pbi.13401 |
(责任编辑 陈丽娥)
 2024, Vol. 51
2024, Vol. 51