文章信息
基金项目
- 国家自然科学基金(32373008);广州市科技计划项目(2023E04J1256);广东省畜禽疫病防治研究重点实验室项目(2023B1212060040);广东省林业局2023年自然资源事务(生态林业建设项目)
作者简介
-
王晓虎,博士,研究员,广东省农业科学院动物卫生研究所人兽共患与牛羊病研究室主任,主要从事动物分子病毒学、野生动物病毒性疫病流行病学及人兽共患传染病学研究,广东省南方现代草牧业(牛)创新团队岗位专家,美国康奈尔大学兽医学院国家公派访问学者,广东省畜牧兽医学会“杰出科技工作者”。近5年主持和参与国家自然科学基金、省市级项目等40余项;以第一或通信作者在国内外期刊发表学术论文30余篇,副主编和参编著作4部;获广东省科学技术二等奖1项、广东省农业技术推广奖3项、广东省农业主推技术4项;获授权国家发明专利5件;参与制定国家标准1项。现为广东省动物防疫技术交流协会监事会监事长、广东省畜牧兽医学会小动物医学分会副会长及兽医诊断与生物制品分会理事。
王晓虎(1979—),男,博士,研究员,研究方向为人兽共患病防控,E-mail:wangxiaohu2020@163.com; 任照文(1994—),男,在读博士生,研究方向为人兽共患病防控,E-mail:670989008@qq.com.
通讯作者
- 陈晶(1982—),女,硕士,副研究员,研究方向为人兽共患病防控,E-mail:chenjing19827@163.com.
文章历史
- 收稿日期:2024-03-11
2. 华南农业大学兽医学院,广东 广州 510642;
3. 西南大学动物医学院,重庆 402460
2. College of Veterinary Medicine, South China Agricultural University, Guangzhou 510642, China;
3. College of Veterinary Medicine, Southwest University, Chongqing 402460, China
中枢神经系统(Central nervous system,CNS)感染是指细菌、病毒和真菌等病原体入侵CNS并在其中定居增殖,能够引起脑膜炎、脑炎和脑脓肿等一系列潜在的致命性疾病。目前,全球每年约有200万人受到CNS感染影响,其中脑膜炎可造成数十万人死亡(http://www.comomeningitis.org),是最致命的疾病之一。由于病死率高且可能导致严重的后遗症,CNS感染已成为全球范围内一项严峻的公共卫生挑战。此外,CNS感染可对畜牧业健康养殖产生巨大的危害。例如,伪狂犬病毒、乙型脑炎病毒和链球菌等病原体感染可导致哺乳仔猪CNS紊乱,致死率高达100%,每年因CNS感染而死亡的仔猪数以万计,全世界因该病所造成的经济损失每年高达几十亿美元[1-4]。为了预防感染,CNS周围由脑膜和脑脊液包裹保护。脑膜从外到内进一步被分为硬脑膜、蛛网膜和软脑膜[5]:软脑膜是一层精细的结构,包覆在脊髓周围;蛛网膜位于软脑膜上方,与含有脑脊液的蛛网膜下腔之间;硬脑膜则是一种坚硬的组织,附着在颅骨上并覆盖在蛛网膜上。一旦病原微生物侵入脑实质,将感染多种细胞,导致神经网络功能障碍及引发炎症,造成局部损伤和细胞死亡[6]。当病原微生物(如细菌和病毒)侵入脑脊液腔时,会造成脑膜炎,引起免疫反应,释放炎症因子如细胞因子,致使局部组织损伤、增加组织通透性并破坏血脑屏障(Blood-brain barrier,BBB)。类似地,脑炎症发生在脑实质中。患有脑膜炎和脑炎的患者通常会在几小时到几天内出现症状,如恶心、呕吐、头痛、光敏感、混乱、发热,甚至丧失意识,若不及时治疗,将导致癫痫、脑积水、认知缺陷甚至死亡[7-8]。
本文将系统介绍BBB结构,并阐述病原微生物穿越BBB进入CNS的具体机制。这不仅可让我们清晰地了解机体中最严格的生物屏障阻碍有害物质入侵CNS的结构基础,也将有助于理解不同病原微生物入侵CNS的方式和机制,揭示一些病原微生物通过利用与宿主的相互作用促进其入侵CNS的巧妙策略,从而为研发新的药物以阻断病原微生物的入侵,或设计新的药物递送系统以治疗CNS感染提供一定的科学依据。
1 血脑屏障(BBB)的细胞和分子结构 1.1 BBB的细胞结构脊椎动物脉管系统表现出高度的器官特异性,其结构和功能可以满足每个组织的特定需求。与外周器官的血管不同,CNS的血管多为连续的无孔结构,具有较为严格的屏障特性,被称为BBB。BBB的历史可以追溯至17世纪末期[9-10],是由多种细胞和细胞外基质等组成的血液循环和脑实质之间紧密而有选择性的界面[11]。BBB能够严格地控制各种小分子化合物、离子和细胞在血液和CNS之间的运动,从而维持脑组织内环境的稳态,使CNS免受病原体、毒素、免疫细胞、炎症和疾病等的损害。
目前,BBB被认为是一种多细胞血管结构,脑微血管内皮细胞(Brain microvessel endothelial cells,BMECs)、周细胞、星形胶质细胞和基底膜(Basement membrane,BM)是其核心元件,周围还附有神经元和小胶质细胞,它们有机地结合在一起,形成神经血管单元(Neurovascular unit,NVU),这是一种将CNS与外周组织分隔开的屏障(图 1)[12]。此外,该屏障还包括如血管周间隙、多糖- 蛋白复合物和诸多酶类等非细胞结构[13]。最终,BBB不仅具有物理意义上的隔离功能,还具有代谢层面上的隔离作用,仅允许分子量低于500 Da的亲脂性分子通过,而限制亲水性分子或高分子量物质进入大脑。
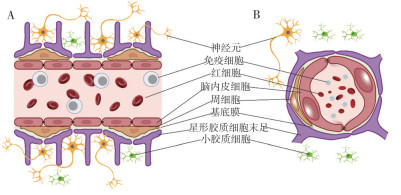
|
| A:脑微血管的矢状面;B:脑微血管的横截面 A: Sagittal schematic diagram of brain microvasculature; B: Cross-sectional schematic diagram of brain microvasculature 图 1 BBB结构示意图 Fig. 1 Diagram of the structure of BBB |
其中,BMECs是BBB的主要组成成分,被认为是BBB的解剖基础,它们形成紧密的脑微血管壁,是血液和脑实质之间的物理屏障。其最重要的特征是相邻的BMECs之间存在相互连接,即紧密连接(Tight junctions,TJs)和粘附连接(Adherens junctions,AJs),这些连接结构形成限制细胞旁路的物理屏障[14-15]。
周细胞属于一种壁细胞,主要存在于CNS的微血管系统中[16],覆盖22%~32% 的内皮表面,并分泌大量能够影响内皮功能的物质,这些物质与微血管结构组装、血管生成及维持新生血管稳定性有关[17-19]。已有研究表明,周细胞分泌的血管生成素可诱导BBB中紧密连接蛋白Occludin的表达,直接参与屏障特性的诱导和维持[20]。
作为哺乳动物脑内分布最广泛的一类细胞,星形胶质细胞因其银染后呈星形而得名[21]。实际上,该细胞的末足覆盖了约99% 的神经血管系统,提供神经元与血管之间的连接,并能够影响BBB的功能,是BBB功能建立的重要参与者[22]。体外共培养实验表明,星形胶质细胞能够上调TJs、转运蛋白和代谢酶的表达,进而增强BBB相关特性[23-25]。
尽管BBB为神经元提供理想且稳定的工作环境,但作为NVU的一部分,神经元对BBB同样具有十分重要的作用。据统计,几乎每个神经元都有与自己互作的毛细血管[26],且一条毛细血管可与多个神经元相连接,当一条毛细血管中血流堵塞时,多个神经元往往同时受到影响[27]。在发育过程中,神经元能够分泌血管内皮生长因子,这是血管形成的关键过程及物质基础。但目前的研究普遍认为,神经元并不是介导血管通透性的主要细胞因素[28-30]。
小胶质细胞也被称为血管周围巨噬细胞,是CNS中的免疫活性细胞,可诱导或抑制脑组织中的先天性和适应性免疫反应。哺乳动物大脑中的小胶质细胞可分为促炎(M1)或抗炎(M2)两种表型[31]。近期研究表明,M1促炎性小胶质细胞通常释放大量的促炎介质,导致BBB功能障碍和通透性增加[32-34]。此外,小胶质细胞还可通过释放活性氧(Reactive oxygen species,ROS)、IL-1a/IL-1b、IL-6、肿瘤坏死因子α(Tumor necrosis factor-α,TNF-α)和基质金属蛋白酶(Matrix metalloproteinases,MMPs)来激活内皮,从而实现对BBB通透性的调控[31, 35-36]。
1.2 BBB的非细胞结构作为NVU的无细胞结构,BM的作用通常被研究者所忽视。实际上,BM是一种高度动态的BBB组成部分,由成分复杂的细胞外基质(Extracellular matrix,ECM)构成,为NVU提供一种结构支撑。研究表明,当内皮与ECM之间的相互作用因整合素β1的缺失而消失时,可观察到内皮间VE-cadherin、CD31和CD99发生大量错位[37],其中,VE-cadherin信号的缺失会导致TJs紊乱,进而造成BBB通透性发生改变[12]。
此外,脑内皮的外表面还存在着一些多糖- 蛋白复合物,主要由蛋白聚糖、糖蛋白和糖胺聚糖(GAG)组成[38]。它们和膜脂质共同形成BMECs的负表面电荷,并通过不同机制影响BBB对分子的通透性[39]。近年来,Kutuzov等[40]更是利用双光子荧光显微镜,观察并记录到内皮表面的多糖- 蛋白复合物能够阻碍40 kDa和150 kDa右旋糖酐在脑微血管处的被动转运情况,而对荧光素钠(376 Da)和Alexa Fluor(643 Da)却无阻挡作用。
2 病原体感染CNS的BBB穿越机制在自然界中,许多微生物能够感染CNS,包括多种细菌、病毒、寄生虫和真菌。随着对嗜神经病原体的深入研究,学者们已经认识到,经过漫长的进化过程,微生物已开发出多种机制以绕过或穿过BBB入侵宿主CNS[41]。目前,嗜神经病原体主要通过5种途径入侵CNS(表 1),包括感染或破坏脑内皮细胞;通过转胞吞作用穿过BBB;以“特洛伊木马”的形式,通过被感染的白细胞浸润而穿过BBB;通过细胞旁间隙进入CNS;以及通过轴突运输由外周绕过BBB进入CNS(图 2)。
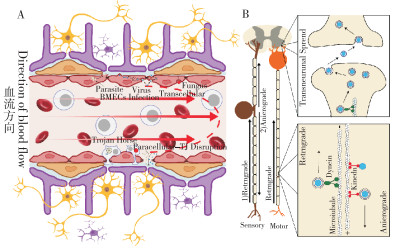
|
| A:不同病原体穿过BBB入侵CNS;B:部分病原体通过轴突转运进入CNS A: Pathogens infiltrating the CNS by crossing the BBB; B: Some pathogens entering the CNS through axonal transport 图 2 病原体通过BBB的神经侵入途径 Fig. 2 Pathways for pathogen neuroinvasion across the BBB |
2.1 感染或破坏脑内皮细胞
尽管脑内皮往往很难被感染,但有几种病原体已被证实可利用脑内皮细胞作为复制的生态位,并通过脑内侧释放子代入侵CNS实质,包括肺炎链球菌、尼帕病毒、寨卡病毒和弓形虫。肺炎链球菌属于链球菌科,是一种经革兰氏染色呈阳性的球菌。研究表明,肺炎链球菌感染BMECs而破坏BBB完整性与其损害机体肺组织的毒力机制相同,肺溶血素似乎在破坏BBB完整性中发挥主要作用[42],这主要是由于肺溶血素能够引起内皮细胞损伤,导致屏障完整性丧失。与肺炎链球菌不同,尼帕病毒(Nipah virus,NiV)是通过感染内皮细胞入侵CNS的病毒性病原体。NiV感染能够引起血管炎,并伴随有内皮细胞损伤、坏死与融合[43, 71]。作为黄病毒科的一员,寨卡病毒(Zika virus,ZIKV)又与NiV不同,它是通过多种细胞表面受体进入BMECs后,并不产生细胞病变效应,也不改变BBB通透性和TJs蛋白的表达[44, 72-73],而是通过复制后出芽或胞吐的方式释放至基底侧,亦或是通过转胞吞作用释放至基底侧[74]。作为专性细胞内寄生性原虫,弓形虫可以在BMECs中复制最终从内侧裂解内皮细胞,使速殖子穿过BBB,对大脑直接进行感染[46]。
2.2 通过BBB的转胞吞作用感染BMECs并复制后,从内侧释放或破坏BMECs并非病原体进入CNS的唯一方式,游离的病原体也可利用宿主细胞的内吞机制实现跨细胞迁移入侵CNS[75]。研究认为,改变细胞表面参与转运或转胞作用蛋白质的位置和数量极有可能是病原体通过转胞吞作用迁移进入脑组织的重要分子机制,也是此类病原体感染期间BBB通透性显著增强的原因之一[76]。例如,肺炎链球菌同样可以通过多种转胞吞作用穿越内皮屏障入侵CNS,包括巨吞饮作用及网格蛋白和小泡介导的胞吞作用[77-78],该过程的分子基础是抑制Mfsd2a的产生和Rho GTP酶的调控[79-80]。黄病毒科成员也能够利用转胞吞作用穿越BBB,特别是西尼罗河病毒(West Nile virus,WNV)、ZIKV和日本乙型脑炎病毒(Japanese encephalitis virus,JEV)[73]。Hasebe等[49]研究表明,复制缺陷型WNV可以从体外极化的BMECs外侧运输至基底侧,成功模拟了病毒在体内从管腔侧转运进入实质的过程。此外,JEV和ZIKV也能够通过小泡介导的BBB转胞吞作用入侵CNS,前者早在1998年就被研究人员通过电子显微镜观察到是利用囊泡穿过脑内皮细胞[50],后者则是通过体外试验发现,病毒从脑内皮细胞的基底侧释放的现象能够被制霉菌素显著减弱,而制霉菌素能够抑制小泡介导的转胞吞作用[44]。
尽管目前尚未发现任何一种寄生虫能够通过转胞吞作用机制进入CNS,但部分真菌已经开发出不依赖于小泡介导转胞吞作用的内吞机制,从而入侵宿主的CNS[81]。比如,多个研究团队已利用基因编辑技术和抑制剂鉴定出多种参与新型隐球菌入侵CNS的宿主蛋白,包括半胱氨酸白三烯和细胞表面的透明质酸糖蛋白受体CD44[82-83]。此外,新型隐球菌还能产生一种专门针对脑内皮的新型分泌性金属蛋白酶Mpr1,并通过该酶诱导新型隐球菌附着于脑内皮细胞表面以促进真菌入侵CNS[52],该过程受到感染期间膜联蛋白A2和S100A10分子的调控[84-85]。
2.3 “特洛伊木马”机制介导的入侵具有吞噬功能的白细胞是机体先天性免疫系统的重要组成部分,它们有助于机体清除病毒、细菌和寄生虫,避免严重的感染。然而,一些病原体能够感染或内化进入白细胞,并利用白细胞的迁移能力穿越BBB,导致CNS感染。在此过程中,白细胞扮演了类似“特洛伊木马”的角色,病原体以此为伪装,骗过机体的免疫系统和生物屏障,从而进入CNS,故该入侵机制被命名为“特洛伊木马”机制。在实际研究中,多种病毒能够通过“特洛伊木马”机制入侵CNS,WNV和NiV便是其中的典范。研究发现,在感染WNV前将小鼠体内的中性粒细胞耗竭可降低病毒血症水平并提高小鼠的存活率[54]。与WNV不同,NiV主要感染未成熟的树突状细胞[86],而被感染的未成熟树突状细胞能够在人BMECs制备的体外BBB模型中迁移,从而导致其他细胞感染,并且其迁移活性显著增加,神经入侵能力提高[55]。
除上述病毒外,顶复门原虫中的弓形虫也可以利用“特洛伊木马”机制入侵CNS[87]。研究表明,弓形虫的神经侵入与单核细胞和树突状细胞的移行存在一定的关系,并且相比于游离的弓形虫速殖子,感染弓形虫的白细胞具有更强的神经侵入能力[88-90]。然而,一些研究人员并不认为弓形虫可以利用“特洛伊木马”机制入侵CNS,主要是因为感染弓形虫的白细胞过继转移给小鼠后并未在CNS中观察到受感染的白细胞[46]。因此,这些研究人员更愿意将有助于弓形虫前往BBB的白细胞称作“便车”。
新型隐球菌同样能通过“特洛伊木马”机制侵入CNS[75]。髓样细胞能够穿越内皮屏障[91],而新型隐球菌通常利用这一机制,通过感染髓样细胞入侵脑膜[92]。已有报道指出,与单独感染新型隐球菌的小鼠相比,注射新型隐球菌感染的单核细胞的小鼠CNS中新型隐球菌的数量显著增多[93]。
2.4 细胞旁路途径细胞旁路途径是指病原体从血液通过内皮细胞间隙扩散到内侧CNS,但该过程受到内皮细胞间TJs的限制[75]。到目前为止,嗜神经病原体已经被证实可通过多种方式破坏TJs,使BBB功能逐渐丧失,从而促进其对CNS的入侵,包括分泌扰乱TJs的蛋白酶和毒素,或利用宿主自身的炎症和免疫反应[75]。已有研究证实,黄病毒科成员WNV的神经侵袭与感染早期BBB通透性增强有关[59]。此外,黄病毒还能通过分泌非结构蛋白1与BMECs结合,诱导组织蛋白酶L和肝素酶的表达,促进内皮糖萼成分的降解,从而破坏BBB完整性,增加其通透性[94]。
除了病毒这类微小的病原微生物外,部分较大的细菌甚至寄生虫也可增加BBB通透性,通过细胞旁路途径入侵CNS。其中,炭疽杆菌通过释放炭疽毒素(InhA和BslA)破坏内皮细胞间TJs,导致BBB通透性增加和出血,进而使炭疽杆菌从血液入侵CNS,并引起致命的出血性脑膜炎[95]。卡氏棘阿米巴原虫则利用其表达的一种甘露糖结合蛋白,促进虫体与脑内皮细胞相互结合,随后通过分泌丝氨酸蛋白酶,以依赖Rho激酶的方式降解TJ蛋白Occludin和ZO-1,最终破坏BBB完整性,导致通透性增加,阿米巴原虫由血液进入CNS[96-97]。
研究显示,弓形虫也可以通过细胞旁路途径进入CNS并导致弓形虫脑炎[61]。弓形虫能够利用肌动蛋白产生“滑翔运动”而跨越BBB,这种运动也被认为是弓形虫跨越小肠上皮细胞的基础[98-99]。体外试验发现,弓形虫能够穿越极化的细胞单层和细胞外基质[100]。弓形虫感染期间还可以直接或间接影响TJs的结构与功能的完整性,进而使其通过细胞旁路途径侵入宿主脑组织中[61]。
2.5 外周神经元的轴突转运轴突转运是指在神经元内部或神经元之间运输物质的过程,它依赖于神经元微管和分子马达,对于神经元的生长、分化、信号传导和可塑性等功能至关重要[101]。轴突运输本是避免血液循环的监测和血脑屏障阻挡,将一些物质绕过BBB送入CNS,但一些病原微生物也能够利用这一绝佳途径入侵CNS并引发感染[102]。体内外试验研究表明,α疱疹病毒可以在神经元和神经元靶点之间进行双向传播,而传播的方向性受特定的病毒基因产物调节[103]。并且,利用电子显微镜观察能够直接在树突突触附近的囊泡结构中观察到WNV的病毒粒子[65, 104-105]。此外,狂犬病毒(Rabies virus,RABV)是被大众所熟知的能够沿轴突逆行传播的病毒,并且RABV轴突运输的单向性已经得到广泛应用,在科学研究中主要用于追踪神经元连接的逆行跨神经元标记物[106]。活体成像的相关研究显示,RABV的轴突逆行转运涉及内体囊泡,以包膜病毒颗粒作为货物在神经元中被携带运输[66]。
3 探索治疗CNS感染的新方法目前,可供CNS感染治疗的选择极为有限,这在很大程度上是因为BBB的结构复杂性、紧密性,以及其功能的特殊性,极大地限制了药物向受感染脑区的输送过程。为研究微生物入侵CNS的分子机制,加快治疗CNS疾病药物的筛选,研究人员目前主要从2个方面入手。一方面,体外构建完善的BBB模型是目前CNS疾病药物筛选所必需的。在过去的20年间,从最简单且应用最广泛的Transwell系统[107]到如今的微流控模型[108]及类器官的出现[109-111],研究人员们所构建的BBB模型越来越接近于实际,这为研究病原微生物入侵CNS机制和筛选脑靶向药物提供了更加真实的环境。此外,近年来,人类NVU芯片的出现,也使得相关研究更加便利[112],例如,NVU芯片已经被用于研究新型隐球菌的神经嗜性及其调控BBB通透性的分子机制[113]。另一方面,研究人员发现,将现有药物与能够穿越BBB的化合物偶联也是一种很有脑递送潜力的方法。特别是在过去的十几年间,许多以短肽为基础的且用于CNS靶向或递送的物质已经被开发出来,例如某种穿梭肽可以很好地增加药物的BBB穿透效率[114-115];狂犬病毒糖蛋白偶联的药物显示出良好的脑靶向作用[116-118]等。通过与现有抗感染药物结合,上述中的大部分物质均有效提高了药物的脑部递送,显示出了良好的治疗效果。目前,其被认为是未来最有希望的CNS感染治疗手段。
综上,构建体外BBB和CNS模型不仅有助于我们了解CNS的生理及其在感染状态下的病理变化,而且有助于筛选治疗微生物感染性脑膜炎的新型抗感染药物。开发可用于脑靶向和BBB转运的物质,是目前开发与制定CNS感染治疗策略的研究重点。
4 结语与展望作为机体内严格的生物屏障之一,BBB阻碍了大部分物质进入CNS,特别是有害物质和病原微生物。然而,病原微生物已找到了穿越BBB入侵CNS的多种途径,并且入侵的过程极其复杂,往往某一种途径被多种病原微生物所利用,同时,病原微生物也能够通过多种途径入侵CNS。那么,病原微生物已经进化出的穿越BBB入侵CNS途径能否对如何治疗CNS疾病带来一些启发吗?答案是肯定的。因此,未来我们仍需要继续研究病原体穿过BBB的机制,以设计新的治疗策略来对抗CNS感染性疾病。
实际上,研究人员已经从病原微生物入侵CNS的机制中探索CNS疾病的治疗策略。最新研究发现,使用狂犬病毒糖蛋白衍生的短肽将小干扰RNA(siRNA)递送到大脑是一种成功的方法,能够有效抑制靶基因的转录表达[118-122]。因此,如果将靶标替换为病原微生物的RNA,那么将有可能在CNS中实现对其复制增殖的干扰,从而发挥治疗效果。另外,“特洛伊木马”入侵途径表明患者自身的免疫细胞可能被病原微生物重新设计[123-124],如果将病原微生物对免疫细胞的改造进行深入研究,人为改造自身免疫细胞,使其成为药物递送入脑的途径将成为可能。类似地,靶向调控介导转胞吞作用的分子如Mfsd2a[125],可以增加小泡的形成,从而利用受体介导的转胞作用进行药物递送。最后,病毒或细菌蛋白可以调控BBB通透性,如黄病毒的NS1蛋白[94, 126]。深入研究这些病毒蛋白的作用机制,将使得人为调控BBB通透性成为可能,实现多种药物渗透进入CNS,特别是那些用于治疗神经感染性疾病的药物。
除了提供治疗机制外,了解病原体进入CNS的途径也有助于防止病原微生物的入侵。许多CNS病原微生物利用细胞表面受体感染神经血管单元的细胞,或通过细胞间的缝隙进入脑和脊髓。深入解析允许神经入侵的结合蛋白可以帮助人类开发小分子抑制剂、抗体或基于免疫的治疗方法,以阻断这些入侵途径。此外,也可以开发针对病原微生物表达的某些特定蛋白的药物,以防止感染或防范嗜神经病原体可能造成的CNS并发症。这种策略对于抵御像炭疽芽孢杆菌之类的病原微生物较为有用。总之,病原体进入CNS的机制是一个持续的研究热点。
| [1] |
LIU Q, KUANG Y, LI Y, GUO H, ZHOU C, GUO S, TAN C, WU B, CHEN H, WANG X. The epidemiology and variation in pseudorabies virus: A continuing challenge to pigs and humans[J]. Viruses, 2022, 14(7): 1463. DOI:10.3390/v14071463 |
| [2] |
孔令达. 我国伪狂犬病现状及伪狂犬病疫苗的应用[J]. 养猪, 2000(1): 38-39. DOI:10.13257/j.cnki.21-1104/s.2000.01.020 KONG L D. Status of pseudorabies in China and application of pseudorabies vaccine[J]. Pig Breeding, 2000(1): 38-39. DOI:10.13257/j.cnki.21-1104/s.2000.01.020 |
| [3] |
FENG Y, ZHANG H, WU Z, WANG S, CAO M, HU D, WANG C. Streptococcus suis infection: An emerging/reemerging challenge of bacterial infectious diseases?[J]. Virulence, 2014, 5(4): 477-497. DOI:10.4161/viru.28595 |
| [4] |
CHAI C, WANG Q, CAO S, ZHAO Q, WEN Y, HUANG X, WEN X, YAN Q, MA X, WU R. Serological and molecular epidemiology of Japanese encephalitis virus infections in swine herds in China, 2006-2012[J]. Journal of Veterinary, 2018, 19(1): 151-155. DOI:10.4142/jvs.2018.19.1.151 |
| [5] |
DASGUPTA K, JEONG J. Developmental biology of the meninges[J]. Genesis, 2019, 57(5): e23288. DOI:10.1002/dvg.23288 |
| [6] |
GIOVANE R A, LAVENDER P D. Central nervous system infections[J]. Primary Care, 2018, 45(3): 505-518. DOI:10.1016/j.pop.2018.05.007 |
| [7] |
SALIMI H, CAIN M D, KLEIN R S. Encephalitic arboviruses: Emergence, clinical presentation, and neuropathogenesis[J]. Neurotherapeutics, 2016, 13(3): 514-534. DOI:10.1007/s13311-016-0443-5 |
| [8] |
THIGPEN M C, WHITNEY C G, MESSONNIER N E, ZELL E R, LYNFIELD R, HADLER J L, HARRISON L H, FARLEY M M, REINGOLD A, BENNETT N M, CRAIG A S, SCHAFFNER W, THOMAS A, LEWIS M M, SCALLAN E, SCHUCHAT A, Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998-2007[J]. New England Journal of Medicine, 2011, 364(21): 2016-2025. DOI:10.1056/NEJMoa1005384 |
| [9] |
RIDLEY H. The anatomy of the brain[M]. Printers: to the Royal Society, 1695.
|
| [10] |
THAKUR J D, SONIG A, CHITTIBOINA P, KHAN I S, WADHWA R, NANDA A. Humphrey ridley (1653 -1708): 17th century evolution in neuroanatomy and selective cerebrovascular injections for cadaver dissection[J]. Neurosurg Focus, 2012, 33(2): E3. DOI:10.3171/2012.6.FOCUS12139 |
| [11] |
徐晓琴. 动物血脑屏障结构及其应用研究的进展[J]. 养殖技术顾问, 2014(5): 258-259. XU X Q. The structure of the blood-brain barrier in animals and advances in its application research[J]. Technical Advisor for Animal Husbandry, 2014(5): 258-259. |
| [12] |
STEWART P A, WILEY M J. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: A study using quail--chick transplantation chimeras[J]. Developmental Biology, 1981, 84(1): 183-192. DOI:10.1016/0012-1606(81)90382-1 |
| [13] |
LANGEN U H, AYLOO S, GU C. Development and cell biology of the blood-brain barrier[J]. Annual Review of Cell and Developmental Biology, 2019, 35: 591-613. DOI:10.1146/annurev-cellbio-100617-062608 |
| [14] |
CZUPALLA C J, LIEBNER S, DEVRAJ K. In vitro models of the blood-brain barrier[J]. Methods in Molecular Biology, 2014, 1135: 415-437. DOI:10.1007/978-1-4939-0320-7_34 |
| [15] |
HASELOFF R F, DITHMER S, WINKLER L, WOLBURG H, BLASIG I E. Transmembrane proteins of the tight junctions at the blood-brain barrier: Structural and functional aspects[J]. Seminars in Cell & Developmental Biology, 2015, 38: 16-25. DOI:10.1016/j.semcdb.2014.11.004 |
| [16] |
BENNETT H C, KIM Y. Pericytes across the lifetime in the central nervous system[J]. Frontiers in Cellular Neuroscience, 2021, 15: 627291. DOI:10.3389/fncel.2021.627291 |
| [17] |
CABELLO-VERRUGIO C. Role of transforming growth factor family of peptides in health and diseases[J]. Current Protein & Peptide Science, 2018, 19(12): 1136-1137. DOI:10.2174/138920371912180926125239 |
| [18] |
FAGIANI E, CHRISTOFORI G. Angiopoietins in angiogenesis[J]. Cancer Let ters, 2013, 328(1): 18-26. DOI:10.1016/j.canlet.2012.08.018 |
| [19] |
HOEBEN A, LANDUYT B, HIGHLEY M S, WILDIERS H, VAN OOSTEROM A T, DE BRUIJN E A. Vascular endothelial growth factor and angiogenesis[J]. Pharmacological Reviews, 2004, 56(4): 549-580. DOI:10.1124/pr.56.4.3 |
| [20] |
M E M A, HE L, NORDLING S, VAZQUEZ-LIEBANAS E, NAHAR K, JUNG B, LI X, TAN B C, CHIN FOO J, CAZENAVE-GASSIOT A, WENK M R, ZARB Y, LAVINA B, QUAGGIN S E, JEANSSON M, GU C, SILVER D L, VANLANDEWIJCK M, BUTCHER E C, KELLER A, BETSHOLTZ C. Single-cell analysis of blood-brain barrier response to pericyte loss[J]. Circulation Research, 2021, 128(4): e46-e62. DOI:10.1161/CIRCRESAHA.120.317473 |
| [21] |
SOFRONIEW M V. Astrocyte reactivity: Subtypes, states, and functions in CNS innate immunity[J]. Trends in Immunology, 2020, 41(9): 758-770. DOI:10.1016/j.it.2020.07.004 |
| [22] |
MATHⅡSEN T M, LEHRE K P, DANBOLT N C, OTTERSEN O P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction[J]. Glia, 2010, 58(9): 1094-1103. DOI:10.1002/glia.20990 |
| [23] |
COLGAN O C, COLLINS N T, FERGUSON G, MURPHY R P, BIRNEY Y A, CAHILL P A, CUMMINS P M. Influence of basolateral condition on the regulation of brain microvascular endothelial tight junction properties and barrier function[J]. Brain Research, 2008, 1193: 84-92. DOI:10.1016/j.brainres.2007.11.072 |
| [24] |
LEE S W, KIM W J, CHOI Y K, SONG H S, SON M J, GELMAN I H, KIM Y J, KIM K W. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier[J]. Nature Medicine, 2003, 9(7): 900-906. DOI:10.1038/nm889 |
| [25] |
MCALLISTER M S, KRIZANAC-BENGEZ L, MACCHIA F, NAFTALIN R J, PEDLEY K C, MAYBERG M R, MARRONI M, LEAMAN S, STANNESS K A, JANIGRO D. Mechanisms of glucose transport at the blood-brain barrier: An in vitro study[J]. Brain Research, 2001, 904(1): 20-30. DOI:10.1016/s0006-8993(01)02418-0 |
| [26] |
ZLOKOVIC B V. Neurovascular mechanisms of Alzheimer's neurodegeneration[J]. Trends in Neurosciences, 2005, 28(4): 202-208. DOI:10.1016/j.tins.2005.02.001 |
| [27] |
OBERMEIER B, VERMA A, RANSOHOFF R M. The blood-brain barrier[J]. Handbook of Clinical Neurology, 2016, 133: 39-59. DOI:10.1016/B978-0-444-63432-0.00003-7 |
| [28] |
KHADK A N, BIKSON M. Neurocapillary-modulation[J]. Neuromodulation, 2022, 25(8): 1299-1311. DOI:10.1111/ner.13338 |
| [29] |
LACOSTE B, COMIN C H, BEN-ZVI A, KAESER P S, XU X, COSTA LDA F, GU C. Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex[J]. Neuron, 2014, 83(5): 1117-1130. DOI:10.1016/j.neuron.2014.07.034 |
| [30] |
RUHRBERG C, BAUTCH V L. Neurovascular development and links to disease[J]. Cellular and Molecular Life Sciences, 2013, 70(10): 1675-1684. DOI:10.1007/s00018-013-1277-5 |
| [31] |
NAYAK D, ROTH T L, MCGAVERN D B. Microglia development and function[J]. Annual Review of Immunology, 2014, 32: 367-402. DOI:10.1146/annurev-immunol-032713-120240 |
| [32] |
ILLES P, RUBINI P, ULRICH H, ZHAO Y, TANG Y. Regulation of microglial functions by purinergic mechanisms in the healthy and diseased CNS[J]. Cells, 2020, 9(5): 1108. DOI:10.3390/cells9051108 |
| [33] |
KEANEY J, CAMPBELL M. The dynamic blood-brain barrier[J]. The Febs Journal, 2015, 282(21): 4067-4079. DOI:10.1111/febs.13412 |
| [34] |
RONALDSON P T, DAVIS T P. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity[J]. Journal of Cerebral Blood Flow and Metabolism, 2020, 40(1_suppl): S6-S24. DOI:10.1177/0271678X20951995 |
| [35] |
CARRANO A, HOOZEMANS J J, VAN DER VIES S M, ROZEMULLER A J, VAN HORSSEN J, DE-VRIES H E. Amyloid beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy[J]. Antioxidants & Redox Signaling, 2011, 15(5): 1167-1178. DOI:10.1089/ars.2011.3895 |
| [36] |
DA FONSECA A C, MATIAS D, GARCIA C, AMARAL R, GERALDO L H, FREITAS C, LIMA F R. The impact of microglial activation on blood-brain barrier in brain diseases[J]. Frontiers in Cellular Neuroscience, 2014, 8: 362. DOI:10.3389/fncel.2014.00362 |
| [37] |
ZOVEIN A C, LUQUE A, TURLO K A, HOFMANN J J, YEE K M, BECKER M S, FASSLER R, MELLMAN I, LANE T F, IRUELA-ARISPE M L. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism[J]. Developmental Cell, 2010, 18(1): 39-51. DOI:10.1016/j.Devcel.2009.12.006 |
| [38] |
WALTER F R, SANTA-MARIA A R, M SZ ROS M, VESZELKA S, D R A, DELI M A. Surface charge, glycocalyx, and blood-brain barrier function[J]. Tissue Barriers, 2021, 9(3): 1904773. DOI:10.1080/21688370.2021.1904773 |
| [39] |
JIN J, FANG F, GAO W, CHEN H, WEN J, WEN X, CHEN J. The structure and function of the glycocalyx and its connection with blood-brain barrier[J]. Frontiers in Cellular Neuroscience, 2021, 15: 739699. DOI:10.3389/fncel.2021.739699 |
| [40] |
KUTUZOV N, FLYVBJERG H, LAURITZEN M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(40): E9429-E9438. DOI:10.1073/pnas.1802155115 |
| [41] |
CAIN M D, SALIMI H, DIAMOND M S, KLEIN R S. Mechanisms of pathogen invasion into the central nervous system[J]. Neuron, 2019, 103(5): 771-783. DOI:10.1016/j.neuron.2019.07.015 |
| [42] |
ZYSK G, SCHNEIDER-WALD B K, HWANG J H, BEJO L, KIM K S, MITCHELL T J, HAKENBECK R, HEINZ H P. Pneumolysin is the main inducer of cytotoxicity to brain microvascular endothelial cells caused by Streptococcus pneumoniae[J]. Infection and Immunity, 2001, 69(2): 845-852. DOI:10.1128/IAI.69.2.845-852.2001 |
| [43] |
WONG K T, SHIEH W J, KUMAR S, NORAIN K, ABDULLAH W, GUARNER J, GOLDSMITH C S, CHUA K B, LAM S K, TAN C T, GOH K J, CHONG H T, JUSOH R, ROLLIN P E, KSIAZEK T G, ZAKI S R, Nipah Virus Pathology Working Group. Nipah virus infection: Pathology and pathogenesis of an emerging paramyxoviral zoonosis[J]. American Journal of Pathology, 2002, 161(6): 2153-2167. DOI:10.1016/S0002-9440(10)64493-8 |
| [44] |
PAPA M P, MEUREN L M, COELHO S V A, LUCAS C G O, MUSTAF Y M, LEMOS MATASSOLI F, SILVEIRA P P, FROST P S, PEZZUTO P, RIBEIRO M R, TANURI A, NOGUEIRA M L, CAMPANATI L, BOZZA M T, PAULA NETO H A, PIMENTEL-COELHO P M, FIGUEIREDO C P, DE-AGUIAR R S, DE-ARRUDA L B. Zika virus infects, activates, and crosses brain microvascular endothelial cells, without barrier disruption[J]. Frontiers in Microbiology, 2017, 8: 2557. DOI:10.3389/fmicb.2017.02557 |
| [45] |
邹松松. HMGB1在日本乙型脑炎病毒入侵中枢神经系统及血脑屏障破坏中的机制研究[D]. 武汉: 华中农业大学, 2023. DOI: 10.27158/d.cnki.ghznu.2022.000098. ZOU S S. The mechanism of HMGB1 involved in central nervous system invasion and blood-brain barrier disruption during Japanese encephalitis virus infection[D]. Wuhan: Huazhong Agricultural University. 2023. DOI: 10.27158/d.cnki.ghznu.2022.000098. |
| [46] |
KONRADT C, UENO N, CHRISTIAN D A, DELONG J H, PRITCHARD G H, HERZ J, BZIK D J, KOSHY A A, MCGAVERN D B, LODOEN M B, HUNTER C A. Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system[J]. Nature Microbiology, 2016, 1: 16001. DOI:10.1038/nmicrobiol.2016.1 |
| [47] |
黄万意. 补体C3/C3a在弓形虫Pru株入侵宿主中枢神经系统中的作用[D]. 广州: 华南农业大学, 2019. DOI: 10.27152/d.cnki.ghanu.2019.001359. HUANG W Y. The molecular mechanism of host's complement component 3/3a in T.gondii invasion of the central nervous system[D]. Guangzhou: South China Agricultural University, 2019. DOI: 10.27152/d.cnki.ghanu.2019.001359. |
| [48] |
IOVINO F, ORIHUELA C J, MOORLAG H E, MOLEMA G, BIJLSMA J J. Interactions between blood-borne Streptococcus pneumoniae and the blood-brain barrier preceding meningitis[J]. PLoS One, 2013, 8(7): e68408. DOI:10.1371/journal.pone.0068408 |
| [49] |
HASEBE R, SUZUKI T, MAKINO Y, IGARASHI M, YAMANOUCHI S, MAEDA A, HORIUCHI M, SAWA H, KIMURA T. Transcellular transport of West Nile virus-like particles across human endothelial cells depends on residues 156 and 159 of envelope protein[J]. BMC Microbiology, 2010, 10: 165. DOI:10.1186/1471-2180-10-165 |
| [50] |
LIOU M L, HSU C Y. Japanese encephalitis virus is transported across the cerebral blood vessels by endocytosis in mouse brain[J]. Cell and Tissue Research, 1998, 293(3): 389-394. DOI:10.1007/s004410051130 |
| [51] |
CHANG Y C, STINS M F, MCCAFFERY M J, MILLER G F, PARE D R, DAM T, PAUL-SATYASEELA M, KIM K S, KWON-CHUNG K J. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier[J]. Infection and Immunity, 2004, 72(9): 4985-4995. DOI:10.1128/IAI.72.9.4985-4995.2004 |
| [52] |
NA-POMBEJRA S, JAMKLANG M, UHRIG J P, VU K, GELLI A. The structure-function analysis of the Mpr1 metalloprotease determinants of activity during migration of fungal cells across the blood-brain barrier[J]. PLoS One, 2018, 13(8): e203020. DOI:10.1371/journal.pone.0203020 |
| [53] |
DREVETS D A, DILLON M J, SCHAWANG J S, VAN ROOIJEN N, EHRCHEN J, SUNDERK TTER C, LEENEN P J. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into the brain during systemic infection of mice[J]. Journal of Immunology, 2004, 172(7): 4418-4424. DOI:10.4049/jimmunol.172.7.4418 |
| [54] |
BAI F, KONG K F, DAI J, QIAN F, ZHANG L, BROWN C R, FIKRIG E, MONTGOMERY R R. A paradoxical role for neutrophils in the pathogenesis of West Nile virus[J]. Journal of Infectious Diseases, 2010, 202(12): 1804-1812. DOI:10.1086/657416 |
| [55] |
TIONG V, SHU M H, WONG W F, ABUBAKAR S, CHANG L Y. Nipah virus infection of immature dendritic cells increases its transendothelial migration across human brain microvascular endothelial cells[J]. Frontiers in Microbiology, 2018, 9: 2747. DOI:10.3389/fmicb.2018.02747 |
| [56] |
UENO N, HARKER K S, CLARKE E V, MCWHORTER F Y, LIU W F, TENNER A J, LODOEN M B. Real-time imaging of Toxoplasma-infected human monocytes under fluidic shear stress reveals rapid translocation of intracellular parasites across endothelial barriers[J]. Cellular Microbiology, 2014, 16(4): 580-595. DOI:10.1111/cmi.12239 |
| [57] |
KAUFMAN-FRANCIS K, DJORDJEVIC J T, JUILLARD P G, LEV S, DESMARINI D, GRAU G E R, SORRELL T C. The early innate immune response to, and phagocyte-dependent entry of, cryptococcus neoformans map to the perivascular space of cortical post-capillary venules in neurocryptococcosis[J]. American Journal of Pathology, 2018, 188(7): 1653-1665. DOI:10.1016/j.ajpath.2018.03.015 |
| [58] |
EBRAHIMI C M, KERN J W, SHEEN T R, EBRAHIMI-FARDOOEE M A, VAN SORGE N M, SCHNEEWIND O, DORAN K S. Penetration of the blood-brain barrier by bacillus anthracis requires the pXO1-encoded BslA protein[J]. Journal of Bacteriology, 2009, 191(23): 7165-7173. DOI:10.1128/JB.00903-09 |
| [59] |
DANIELS B P, HOLMAN D W, CRUZ-ORENGO L, JUJJAVARAPU H, DURRANT D M, KLEIN R S. Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals[J]. mBio, 2014, 5(5): e1414-e1476. DOI:10.1128/mBio.01476-14 |
| [60] |
ALSAM S, SISSONS J, JAYASEKERA S, KHAN N A. Extracellular proteases of Acanthamoeba castellanii (encephalitis isolate belonging to T1 genotype) contribute to increased permeability in an in vitro model of the human blood-brain barrier[J]. Journal of Infection, 2004, 51(2): 150-156. DOI:10.1016/j.jinf.2004.09.001 |
| [61] |
ROSS E C, OLIVERA G C, BARRAGAN A. Early passage of Toxoplasma gondii across the blood-brain barrier[J]. Trends in Parasitology, 2022, 38(6): 450-461. DOI:10.1016/j.pt.2022.02.003 |
| [62] |
DRAMSI S, L VI S, TRILLER A, COSSART P. Entry of Listeria monocytogenes into neurons occurs by cell-to-cell spread: An in vitro study[J]. Infection and Immunity, 1998, 66(9): 4461-4468. DOI:10.1128/IAI.66.9.4461-4468.1998 |
| [63] |
ANTINONE S E, SMITH G A. Retrograde axon transport of herpes simplex virus and pseudorabies virus: A live-cell comparative analysis[J]. Journal of Virology, 2010, 84(3): 1504-1512. DOI:10.1128/JVI.02029-09 |
| [64] |
MIRANDA-SAKSENA M, DENES C E, DIEFENBACH R J, CUNNINGHAM A L. Infection and transport of herpes simplex virus type 1 in neurons: Role of the cytoskeleton[J]. Viruses, 2018, 10(2): 92. DOI:10.3390/v10020092 |
| [65] |
SAMUEL M A, WANG H, SIDDHARTHAN V, MORREY J D, DIAMOND M S. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(43): 17140-17145. DOI:10.1073/pnas.0705837104 |
| [66] |
KLINGEN Y, CONZELMANN K K, FINKE S. Double-labeled rabies virus: Live tracking of enveloped virus transport[J]. Journal of Virology, 2008, 82(1): 237-245. DOI:10.1128/JVI.01342-07 |
| [67] |
OHKA S, SAKAI M, BOHNERT S, IGARASHI H, DEINHARDT K, SCHIAVO G, NOMOTO A. Receptor-dependent and -independent axonal retrograde transport of poliovirus in motor neurons[J]. Journal of Virology, 2009, 83(10): 4995-5004. DOI:10.1128/JVI.02225-08 |
| [68] |
ROUSSARIE J P, RUFFIE C, BRAHIC M. The role of myelin in Theiler's virus persistence in the central nervous system[J]. PLoS Pathogens, 2007, 3(2): e23. DOI:10.1371/journal.ppat.0030023 |
| [69] |
BEIER K T, BORGHUIS B G, EL-DANAF R N, HUBERMAN A D, DEMB J B, CEPKO C L. Transsynaptic tracing with vesicular stomatitis virus reveals novel retinal circuitry[J]. Journal of Neuroscience, 2013, 33(1): 35-51. DOI:10.1523/JNEUROSCI.0245-12.2013 |
| [70] |
ENCALADA S E, SZPANKOWSKI L, XIA C H, GOLDSTEIN L S. Stable kinesin and dynein assemblies drive the axonal transport of mammalian prion protein vesicles[J]. Cell, 2011, 144(4): 551-565. DOI:10.1016/j.cell.2011.01.021 |
| [71] |
FREITAG T C, MAISNER A. Early activation of primary brain microvascular endothelial cells by nipah virus glycoprotein-containing particles[J]. Journal of Virology, 2015, 90(5): 2706-2709. DOI:10.1128/JVI.02825-15 |
| [72] |
RICHARD A S, SHIM B S, KWON Y C, ZHANG R, OTSUKA Y, SCHMITT K, BERRI F, DIAMOND M S, CHOE H. AXL-dependent infection of human fetal endothelial cells distinguishes Zika virus from other pathogenic flaviviruses[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(8): 2024-2029. DOI:10.1073/pnas.1620558114 |
| [73] |
陈浩威, 崔旻. 黄病毒入侵中枢神经系统的分子机制研究进展[J]. 华中农业大学学报, 2021, 40(4): 85-93. DOI:10.13300/j.cnki.hnlkxb.2021.04.011 CHEN H W, CUI M. Research progress on molecular mechanism of flavivirus invading central nervous system[J]. Journal of Huazhong Agricultural University, 2021, 40(4): 85-93. DOI:10.13300/j.cnki.hnlkxb.2021.04.011 |
| [74] |
CHIU C F, CHU L W, LIAO I C, SIMANJUNTAK Y, LIN Y L, JUAN C C, PING Y H. The mechanism of the zika virus crossing the placental barrier and the blood-brain barrier[J]. Frontiers in Microbiology, 2020, 11: 214. DOI:10.3389/fmicb.2020.00214 |
| [75] |
LE GOVIC Y, DEMEY B, CASSEREAU J, BAHN Y S, PAPON N. Pathogens infecting the central nervous system[J]. PLoS Pathogens, 2022, 18(2): e1010234. DOI:10.1371/journal.ppat.1010234 |
| [76] |
VILLASE OR R, LAMPE J, SCHWANINGER M, COLLIN L. Intracellular transport and regulation of transcytosis across the blood-brain barrier[J]. Cellular and Molecular Life Sciences, 2019, 76(6): 1081-1092. DOI:10.1007/s00018-018-2982-x |
| [77] |
GRADSTEDT H, IOVINO F, BI JLSMA J J. Streptococcus pneumoniae invades endothelial host cells via multiple pathways and is killed in a lysosome dependent manner[J]. PLoS One, 2013, 8(6): e65626. DOI:10.1371/journal.pone.0065626 |
| [78] |
LOH L N, GAO G, TUOMANEN E I. Dissecting bacterial cell wall entry and signaling in eukaryotic cells: An actin-dependent pathway parallels platelet-activating factor receptor-mediated endocytosis[J]. mBio, 2017, 8(1): e02030-16. DOI:10.1128/mBio.02030-16 |
| [79] |
AL-OBAIDI M, DESA M. Mechanisms of blood brain barrier disruption by different types of bacteria, and bacterial-host interactions facilitate the bacterial pathogen invading the brain[J]. Cellular and Molecular Neurobiology, 2018, 38(7): 1349-1368. DOI:10.1007/s10571-018-0609-2 |
| [80] |
DEL P M, LOLO F N, ECHARRI A. Caveolae: Mechanosensing and mechanotransduction devices linking membrane trafficking to mechanoadaptation[J]. Current Opinion in Cell Biology, 2021, 68: 113-123. DOI:10.1016/j.ceb.2020.10.008 |
| [81] |
GORALSKA K, BLASZKOWSKA J, DZIKOWIEC M. Neuroinfections caused by fungi[J]. Infection, 2018, 46(4): 443-459. DOI:10.1007/s15010-018-1152-2 |
| [82] |
JONG A, WU C H, GONZALES-GOMEZ I, KWON-CHUNG K J, CHANG Y C, TSENG H K, CHO W L, HUANG S H. Hyaluronic acid receptor CD44 deficiency is associated with decreased Cryptococcus neoformans brain infection[J]. Journal of Biological Chemistry, 2012, 287(19): 15298-15306. DOI:10.1074/jbc.M112.353375 |
| [83] |
ZHU L, MARUVADA R, SAPIRSTEIN A, PETERS-GOLDEN M, KIM K S. Cysteinyl leukotrienes as novel host factors facilitating Cryptococcus neoformans penetration into the brain[J]. Cellular Microbiology, 2017, 19(3): 10. DOI:10.1111/cmi.12661 |
| [84] |
NA-POMBEJRA S, SALEMI M, PHINNEY B S, GELLI A. The metalloprotease, mpr1, engages annexina2 to promote the transcytosis of fungal cells across the blood-brain barrier[J]. Frontiers in Cellular and Infection Microbiology, 2017, 7: 296. DOI:10.3389/fcimb.2017.00296 |
| [85] |
CHEN Y, CHEN J, WEN H, GAO P, WANG J, ZHENG Z, GU J. S100A10 downregulation inhibits the phagocytosis of Cryptococcus neoformans by murine brain microvascular endothelial cells[J]. Microbial Pathogenesis, 2011, 51(3): 96-100. DOI:10.1016/j.micpath.2011.05.003 |
| [86] |
MATHIEU C, POHL C, SZECSI J, TRAJKOVIC-BODENNEC S, DEVERGNAS S, RAOUL H, COSSET FL, GERLIER D, WILD T F, HORVAT B. Nipah virus uses leukocytes for efficient dissemination within a host[J]. Journal of Virology, 2011, 85(15): 7863-7871. DOI:10.1128/JVI.00549-11 |
| [87] |
MENDEZ O A, KOSHY A A. Toxoplasma gondii: Entry, association, and physiological influence on the central nervous system[J]. PLoS Pathogens, 2017, 13(7): e1006351. DOI:10.1371/journal.ppat.1006351 |
| [88] |
OLAFSSON E B, BARRAGAN A. The unicellular eukaryotic parasite Toxoplasma gondii hijacks the migration machinery of mononuclear phagocytes to promote its dissemination[J]. Biology of the Cell, 2020, 112(9): 239-250. DOI:10.1111/boc.202000005 |
| [89] |
SCHNEIDER C A, FIGUEROA VELEZ D X, AZEVEDO R, HOOVER E M, TRAN C J, LO C, VADPEY O, GANDHI S P, LODOEN M B. Imaging the dynamic recruitment of monocytes to the blood-brain barrier and specific brain regions during Toxoplasma gondii infection[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(49): 24796-24807. DOI:10.1073/pnas.1915778116 |
| [90] |
UNNO A, SUZUKI K, XUAN X, NISHIKAWA Y, KITOH K, TAKASHIMA Y. Dissemination of extracellular and intracellular Toxoplasma gondii tachyzoites in the blood flow[J]. Parasitology International, 2008, 57(4): 515-518. DOI:10.1016/j.parint.2008.06.004 |
| [91] |
AMANN L, MASUDA T, PRINZ M. Mechanisms of myeloid cell entry to the healthy and diseased central nervous system[J]. Nature Immunology, 2023, 24(3): 393-407. DOI:10.1038/s41590-022-01415-8 |
| [92] |
SANTIAGO-TIRADO F H, ONKEN M D, COOPER J A, KLEIN R S, DOERING T L. Trojan horse transit contributes to blood-brain barrier crossing of a eukaryotic pathogen[J]. mBio, 2017, 8(1): e02183-16. DOI:10.1128/mBio.02183-16 |
| [93] |
CHARLIER C, NIELSEN K, DAOU S, BRIGITTE M, CHRETIEN F, DROMER F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans[J]. Infection and Immunity, 2009, 77(1): 120-127. DOI:10.1128/IAI.01065-08 |
| [94] |
PUERTA-GUARDO H, GLASNER D R, ESPINOSA D A, BIERING S B, PATANA M, RATNASIRI K, WANG C, BEATTY P R, HARRIS E. Flavivirus NS1 triggers tissue-specific vascular endothelial dysfunction reflecting disease tropism[J]. Cell Reports, 2019, 26(6): 1598-1613. DOI:10.1016/j.celrep.2019.01.036 |
| [95] |
MUKHERJEE D V, TONRY J H, KIM K S, RAMARAO N, POPOVA T G, BAILEY C, POPOV S, CHUNG M C. Bacillus anthracis protease InhA increases blood-brain barrier permeability and contributes to cerebral hemorrhages[J]. PLoS One, 2011, 6(3): e17921. DOI:10.1371/journal.pone.0017921 |
| [96] |
ALSAM S, KIM K S, STINS M, RIVAS A O, SISSONS J, KHAN N A. Acanthamoeba interactions with human brain microvascular endothelial cells[J]. Microbial Pathogenesis, 2003, 35(6): 235-241. DOI:10.1016/j.micpath.2003.07.001 |
| [97] |
SIDDIQUI R, EMES R, ELSHEIKHA H, KHAN N A. Area 51: How do Acanthamoeba invade the central nervous system?[J]. Trends in Parasitology, 2011, 27(5): 185-189. DOI:10.1016/j.pt.2011.01.005 |
| [98] |
DOBROWOLSKI J M, SIBLEY L D. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite[J]. Cell, 1996, 84(6): 933-939. DOI:10.1016/s0092-8674(00)81071-5 |
| [99] |
TARDIEUX I, MENARD R. Migration of Apicomplexa across biological barriers: The Toxoplasma and Plasmodium rides[J]. Traffic, 2008, 9(5): 627-635. DOI:10.1111/j.1600-0854.2008.00703.x |
| [100] |
BARRAGAN A, SIBLEY L D. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence[J]. Journal of Experimental Medicine, 2002, 195(12): 1625-1633. DOI:10.1084/jem.20020258 |
| [101] |
OKABE S, HIROKAWA N. Axonal transport[J]. Current Opinion in Cell Biology, 1989, 1(1): 91-97. DOI:10.1016/s0955-0674(89)80043-2 |
| [102] |
TAYLOR M P, ENQUIST L W. Axonal spread of neuroinvasive viral infections[J]. Trends in Microbiology, 2015, 23(5): 283-288. DOI:10.1016/j.tim.2015.01.002 |
| [103] |
SMITH G. Herpesvirus transport to the nervous system and back again[J]. Annual Review of Microbiology, 2012, 66: 153-176. DOI:10.1146/annurev-micro-092611-150051 |
| [104] |
HUNSPERGER E A, ROEHRIG J T. Temporal analyses of the neuropathogenesis of a West Nile virus infection in mice[J]. Journal of Neurovirology, 2006, 12(2): 129-139. DOI:10.1080/13550280600758341 |
| [105] |
MAXIMOVA O A, BERNBAUM J G, PLETNEV A G. West Nile virus spreads transsynaptically within the pathways of motor control: Anatomical and ultrastructural mapping of neuronal virus infection in the primate central nervous system[J]. PLoS Neglected Tropical Diseases, 2016, 10(9): e4980. DOI:10.1371/journal.pntd.0004980 |
| [106] |
UGOLINI G. Rabies virus as a transneuronal tracer of neuronal connections[J]. Advances in Virus Research, 2011, 79: 165-202. DOI:10.1016/B978-0-12-387040-7.00010-X |
| [107] |
SU Y, SINKO P J. Dr ug deliver y across the blood-brain barrier: Why is it difficult? how to measure and improve it?[J]. Expert Opinion on Drug Discovery, 2006, 3(3): 419-435. DOI:10.1517/17425247.3.3.419 |
| [108] |
SUN X Y, JU X C, LI Y, ZENG P M, WU J, ZHOU Y Y, SHEN L B, DONG J, CHEN Y J, LUO Z G. Generation of vascularized brain organoids to study neurovascular interactions[J]. Elife, 2022, 11: e76707. DOI:10.7554/eLife.76707 |
| [109] |
PENG B, HAO S, TONG Z, BAI H, PAN S, LIM K L, LI L, VOELCKER N H, HUANG W. Blood-brain barrier (BBB)-on-a-chip: A promising breakthrough in brain disease research[J]. Lab on a Chip, 2022, 22(19): 3579-3602. DOI:10.1039/d2lc00305h |
| [110] |
CAMPISI M, SHIN Y, OSAKI T, HAJAL C, CHIONO V, KAMM R D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes[J]. Biomaterials, 2018, 180: 117-129. DOI:10.1016/j.biomaterials.2018.07.014 |
| [111] |
ODDO A, PENG B, TONG Z, WEI Y, TONG W Y, THISSEN H, VOELCKER N H. Advances in microfluidic blood-brain barrier (BBB) models[J]. Trends in Biotechnology, 2019, 37(12): 1295-1314. DOI:10.1016/j.tibtech.2019.04.006 |
| [112] |
MAOZ B M. Brain-on-a-chip: Characterizing the next generation of advanced in vitro platforms for modeling the central nervous system[J]. APL Bioengineering, 2021, 5(3): 30902. DOI:10.1063/5.0055812 |
| [113] |
KIM J, LEE K T, LEE J S, SHIN J, CUI B, YANG K, CHOI Y S, CHOI N, LEE S H, LEE J H, BAHN Y S, CHO S W. Fungal brain infection modelled in a human-neurovascular-unit-on-a-chip with a functional blood-brain barrier[J]. Nature Biomedical Engineering, 2021, 5(8): 830-846. DOI:10.1038/s41551-021-00743-8 |
| [114] |
MALAKOUTIKHAH M, GUIXER B, ARRANZ-GIBERT P, TEIXID M, GIRALT E. 'A la carte' peptide shuttles: Tools to increase their passage across the blood-brain barrier[J]. ChemMedChem, 2014, 9(7): 1594-1601. DOI:10.1002/cmdc.201300575 |
| [115] |
MENDON A D A, BAKKER M, CRUZ-OLIVEIRA C, NEVES V, JIM NEZ M A, DEFAUS S, CAVACO M, VEIGA A S, CADIMA-COUTO I, CASTANHO M A R B, ANDREU D, TODOROVSKI T. Penetrating the blood-brain barrier with new peptide-porphyrin conjugates having anti-HIV activity[J]. Bioconjugate Chemistry, 2021, 32(6): 1067-1077. DOI:10.1021/acs.bioconjchem.1c00123 |
| [116] |
ZHANG L, WU T, SHAN Y, LI G, NI X, CHEN X, HU X, LIN L, LI Y, GUAN Y, GAO J, CHEN D, ZHANG Y, PEI Z, CHEN X. Therapeutic reversal of Huntington's disease by in vivo self-assembled siRNAs[J]. Brain, 2021, 144(11): 3421-3435. DOI:10.1093/brain/awab354 |
| [117] |
CHENG W, SU Y L, HSU H H, LIN Y H, CHU L A, HUANG W C, LU Y J, CHIANG C S, HU S H. Rabies virus glycoprotein-mediated transportation and T cell infiltration to brain tumor by magnetoelectric gold yarnballs[J]. American Chemical Society, 2022, 16(3): 4014-4027. DOI:10.1021/acsnano.1c09601 |
| [118] |
HUEY R, HAWTHORNE S, MCCARRON P. The potential use of rabies virus glycoprotein-derived peptides to facilitate drug delivery into the central nervous system: A mini review[J]. Journal of Drug Targeting, 2017, 25(5): 379-385. DOI:10.1080/1061186X.2016.1223676 |
| [119] |
GAO Y, WANG Z Y, ZHANG J, ZHANG Y, HUO H, WANG T, JIANG T, WANG S. RVG-peptide-linked trimethylated chitosan for delivery of siRNA to the brain[J]. Biomacromolecules, 2014, 15(3): 1010-1018. DOI:10.1021/bm401906p |
| [120] |
KIM S S, YE C, KUMAR P, CHIU I, SUBRAMANYA S, WU H, SHANKAR P, MANJUNATH N. Targeted delivery of siRNA to macrophages for anti-inflammatory treatment[J]. Molecular Therapy, 2010, 18(5): 993-1001. DOI:10.1038/mt.2010.27 |
| [121] |
KUMAR P, WU H, MCBRIDE J L, JUNG K E, KIM M H, DAVIDSON B L, LEE S K, SHANKAR P, MANJUNATH N. Transvascular delivery of small interfering RNA to the central nervous system[J]. Nature, 2007, 448: 39-43. DOI:10.1038/nature05901 |
| [122] |
苏峰, 熊峰, 曹俊如, 储晓琴, 何广卫, 尹莉芳. 基于穿透血脑屏障的siRNA纳米递药系统研究进展[J]. 中国药学杂志, 2024, 59(4): 285-295. SU F, XIONG F, CAO J R, CHU X Q, HE G W, YI L F. Advances of siRNA nano-delivery system based on penetration of blood-brain barrier[J]. Chinese Pharmaceutical Journal, 2024, 59(4): 285-295. |
| [123] |
MITCHELL M J, BILLINGSLEY M M, HALEY R M, WECHSLER M E, PEPPAS N A, LANGER R. Engineering precision nanoparticles for dr ug deliver y[J]. Nature Reviews Dr ug Discover y, 2021, 20(2): 101-124. DOI:10.1038/s41573-020-0090-8 |
| [124] |
NIELAND L, MAHJOUM S, GRANDELL E, BREYNE K, BREAKEFIELD X O. Engineered EVs designed to target diseases of the CNS[J]. Journal of Controlled Release, 2023, 356: 493-506. DOI:10.1016/j.jconrel.2023.03.009 |
| [125] |
ANDREONE B J, CHOW B W, TATA A, LACOSTE B, BEN-ZVI A, BULLOCK K, DEIK A A, GINTY D D, CLISH C B, GU C. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis[J]. Neuron, 2017, 94(3): 581-594. DOI:10.1016/j.neuron.2017.03.043 |
| [126] |
LI F, WANG Y, YU L, CAO S, WANG K, YUAN J, WANG C, WANG K, CUI M, FU Z F. Viral infection of the central nervous system and neuroinflammation precede blood-brain barrier disruption during Japanese encephalitis virus infection[J]. Journal of Virology, 2015, 89(10): 5602-5614. DOI:10.1128/JVI.00143-15 |
(责任编辑 马春敏)
 2024, Vol. 51
2024, Vol. 51