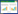文章信息
基金项目
- 国家自然科学基金(U20A2065)
作者简介
- 张霄(1994—),女,在读硕士生,研究方向为水生动物病害防控,E-mail: Bztsgqjx@163.com.
通讯作者
- 蔡佳(1982—),男,博士,副教授,研究方向为水生动物病害防控,E-mail: matrix924@foxmail.com.
文章历史
- 收稿日期:2024-03-15
2. 广西科学院/广西水产生物技术与现代生态养殖重点实验室,广西 南宁 530007
2. Guangxi Academy of Sciences/Guangxi Key Laboratory of Aquatic Biotechnology and Modern Ecological Aquaculture, Nanning 530007, China
【研究意义】卵形鲳鲹(Trachinotus ovatus)又称金鲳、鲳鲹等,鲈形目鲹科鲳鲹属,是我国主要的海洋经济鱼类[1]。由于养殖密度不断提高和养殖环境的恶化,卵形鲳鲹病害频发,导致严重的经济损失[2]。卵形鲳鲹病害主要分为病毒性病害、细菌性病害和寄生虫。目前,国内外已报道的卵形鲳鲹病毒性病害主要为脑神经坏死病毒病;引起细菌性病害的病原菌有创伤弧菌(Vibrio vulnificus)、溶藻弧菌(V. alginolyticus)、副溶血弧菌(V. parahemolyticus)、鳗弧菌(V. anguillarum)和假单孢菌(Pseudomonas);寄生虫主要有指环虫(Dactylogyrus)、瓣体虫(Petalosoma)、车轮虫(Trichodina)和刺激隐核虫(Cryptocaryon irritans)[3–7]。卵形鲳鲹病害主要以预防为主,通过在饲料中添加免疫增强剂等增强卵形鲳鲹对细菌或病毒的抵抗力[8]。研究表明,在饵料中添加大豆异黄酮可改善卵形鲳鲹的生长性能和先天免疫能力,提升其对哈维弧菌(V. harveyi)的抵抗力[9];喂食β- 葡聚糖可有效改善卵形鲳鲹肠道微生物多样性,降低肠道内弧菌数量[10]。但利用益生菌防控卵形鲳鲹疾病的研究尚未见报道。【前人研究进展】抗生素和化学药物是水产养殖中传统的病害防控方式,但长期使用抗生素和化学药物会造成环境污染、加速细菌产生耐药性[11]。研究表明,抗生素治疗与鱼类肠道菌群紊乱具有相关性,抗生素可对肠道菌群产生长期的影响[12]。为减少抗生素和化学药物的使用,实现可持续的健康养殖模式,可替代抗生素用于鱼病防控的疫苗、益生菌、益生元、免疫调节剂等越来越受关注。益生菌可以改善水产养殖品种的生长性能、免疫力、肠道微生物多样性和养殖水质等,且有高效、环保等优点,具有广阔的应用前景[13]。近年来,芽孢杆菌、肠球菌、乳酸杆菌等多种益生菌已广泛应用于水产养殖中[14]。在幼鱼阶段应用益生菌可带来更优良的效益[15],在养殖水体中添加复合益生菌可提高罗非鱼成活率、改善养殖环境[16]。Reda等[17]从罗非鱼肠道中分离出粪肠球菌等益生菌,并评估不同菌株在水产养殖中的潜在用途。益生菌的推广使用不仅可以提高水产养殖业生产效益,还有助于提高水产品质量安全,具有良好的应用价值。【本研究切入点】益生菌能维持水产经济动物肠道菌群稳定增强免疫力、改善养殖水质。但是适用于卵形鲳鲹养殖的益生菌制剂的研究还相对较少,卵形鲳鲹体内是否存在益生菌还有待探究。【拟解决的关键问题】本研究从健康卵形鲳鲹肠道中分离出粪肠球菌,通过菌株生理生化鉴定,检测其耐药性、耐盐能力、毒力基因等方面的特性,为卵形鲳鲹益生菌制剂的开发奠定基础。
1 材料与方法 1.1 试验材料供试卵形鲳鲹于2023年11月购自湛江某水产批发市场。
培养基及主要试剂:德氏乳杆菌(MRS)培养基、酵母浸出粉胨葡萄糖(YPD)琼脂培养基购自广东环凯微生物科技有限公司;细菌基因组DNA提取试剂盒购自天根生化科技(北京)有限公司;抗菌药物药敏纸片购自杭州微生物试剂有限公司;生化鉴定试剂条购自青岛海博生物技术有限公司;革兰染色试剂盒购自南京建成生物工程研究所。
1.2 试验方法1.2.1 菌株分离与鉴定 在无菌条件下将卵形鲳鲹肠道内容物划线接种于YPD琼脂培养基上,37℃倒置培养12~24 h,观察菌落的形态特征。用接种环挑取单菌落进行涂片和革兰氏染色,并在在光学显微镜下观察其形态和革兰氏染色结果。
1.2.2 DNA提取、16S rRNA基因序扩增和构建系统进化树 挑取典型的单菌落,接种于MRS液体培养基,37 ℃培养12 h,使用细菌基因组DNA提取试剂盒提取DNA,以基因组DNA为模板,使用通用引物27F(5'-AGAGTTTGATCCTGGCTCAG-3')和1492R(5'-ACGGCTACCTTGTTACGACTT-3')[18]通过PCR扩增分离菌株的16S rRNA基因。PCR反应体系为50 μL:上、下游引物各1 μL,DNA模板1 μL,ddH2O 24 μL,2x sanTaq PCR Master Mix 23 μL,以ddH2O作为阴性对照。反应条件:95℃预变性3 min;95 ℃变性30 s,55 ℃退火30 s, 72 ℃ 1 min,35个循环;72 ℃延伸5 min。PCR产物经1.0% 琼脂糖凝胶电泳检测后,将阳性PCR产物送往上海生工生物技术服务有限公司进行测序。将测序结果在NCBI数据库进行比对,采用MEGA 5.0进行系统进化分析。
1.2.3 生理生化特性鉴定 在无菌条件下使用接种环挑取纯化后的分离菌株单菌落,接种至2 mL无菌生理盐水管中,充分研磨混匀,制成0.5麦氏浊度的均一菌悬液,后续操作按照乳酸菌生化鉴定试剂条说明书进行。
1.2.4 生长曲线、产酸曲线的测定 取活化的分离菌株按照1∶100(OD600=0.5)的接种量比例接种至200 mL MRS液体培养基中,37 ℃ 150 r/min摇床培养,每隔2 h测定菌液的OD600和pH值,分别绘制其生长曲线和产酸曲线,以无菌MRS液体培养基作为对照,3次重复。
1.2.5 药敏试验 取100 μL分离菌株的菌液均匀涂布在YPD琼脂培养基上、晾干,采用K-B纸片扩散法,用镊子将抗菌药物药敏纸片贴在培养基表面,37℃恒温培养24 h,每组3次重复。用游标卡尺测量抑菌圈的大小,按照美国临床实验室标准化委员会(CLSI)标准进行分离菌株的药物敏感性观测和鉴定[19]。
1.2.6 毒力基因检测 选取8个主要毒力基因:黏附素基因efaA和as、胶原结合蛋白基因ace、表面蛋白基因esp、生物膜基因agg、抗生素抗性基因BlaTEM、毒力因子基因fsrA,溶血素基因cylA进行检测,引物(表 1)设计参照文献[20-24],以菌株DNA为模板,PCR反应体系见1.2.2。毒力基因as、fsrA、cylA的PCR反应条件为:94℃ 5 min;94℃变性1 min,53℃退火30 s,72℃延伸2 min,30个循环;72℃延伸10 min。毒力基因efaA、esp、agg等的PCR反应条件为:94℃ 5 min;94℃变性1 min,56℃退火30 s,72℃延伸2 min,30个循环;72℃延伸10 min。使用1% 琼脂糖凝胶检测电泳条带,使用凝胶成像仪观察记录。
1.2.7 耐盐能力试验 取活化的分离菌株按照1∶100(OD600=0.5)的接种量比例依次接种到含有0%、0.4%、3.5%、5%、8%、12%、16% NaCl的50 mL MRS液体培养基中,37℃ 150 r/min摇床培养24 h,每隔2 h取样,测定OD600值。
2 结果与分析 2.1 分离菌株的形态学观察分离菌株在YPD琼脂培养基上的菌落呈淡灰白色、圆形、扁平微隆起、边缘整齐、表面湿润光滑,培养20~24 h后单菌落直径约1 mm。分离菌株革兰氏染色呈阳性,镜检观察其形状为圆形或卵圆形、单个或成双排列的球菌(图 1)。
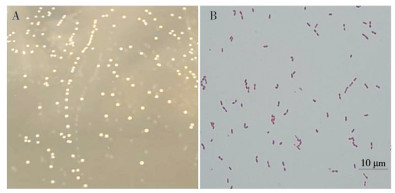
|
| 图 1 分离菌株的菌落形态(A)和革兰氏染色结果(B) Fig. 1 Colony morphology (A) and Gram staining results (B) of isolated strains |
2.2 16S rRNA PCR扩增与系统发育树
分离菌株的16S rRNA PCR产物经琼脂糖凝胶电泳后可在1 500 bp左右检测到条带。将测序结果与参考菌株进行比对,构建系统发育树。结果(图 2)显示,分离菌株属于粪肠球菌(Enterococcus faecalis),暂命名为菌株SZ07。
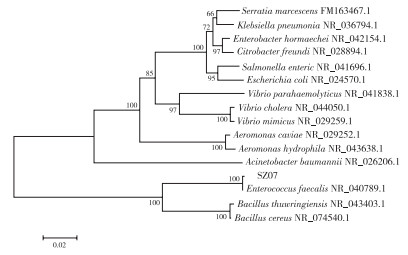
|
| 图 2 基于16S rRNA基因构建的菌株SZ07系统发育树 Fig. 2 Phylogenetic tree constructed based on 16S rRNA gene sequence of isolated strain SZ07 |
2.3 菌株SZ07生理生化鉴定结果
使用七叶苷、纤维二糖、麦芽糖、甘露醇、水杨苷、山梨醇、蔗糖、棉子糖、菊糖、乳糖10种生化管对菌株SZ07进行生理生化鉴定,以王佰涛等[25]筛选到的粪肠球菌菌株SWS50为参照。结果(表 2)显示菌株SZ07的七叶苷、纤维二糖、麦芽糖、甘露醇、水杨苷、山梨醇、蔗糖、菊糖、乳糖试验为阳性,棉子糖试验为阴性,菌株SWS50表现一致,表明菌株SZ07为粪肠球菌。
2.4 菌株SZ07生长曲线和产酸曲线测定结果
由图 3A可知,培养4 h后,菌株SZ07的OD600值快速上升,菌株大量繁殖,进入对数期;培养12 h后菌株进入生长稳定期,活菌数变化较小。由图 3B可知,菌株SZ07培养4 h后产酸能力增强,可产生大量有机酸,使菌液pH值快速下降;培养12 h开始菌液pH值趋于稳定。表明粪肠球菌可通过产酸降低宿主肠道内pH,营造促进其他有益乳酸菌生长繁殖且抑制病原性细菌生长的环境。
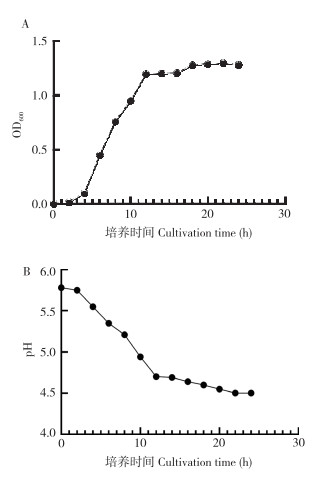
|
| 图 3 菌株SZ07的生长曲线(A)和产酸能力(B) Fig. 3 Growth curve (A) and acid production capacity (B) of strain SZ07 |
2.5 菌株SZ07药敏试验结果
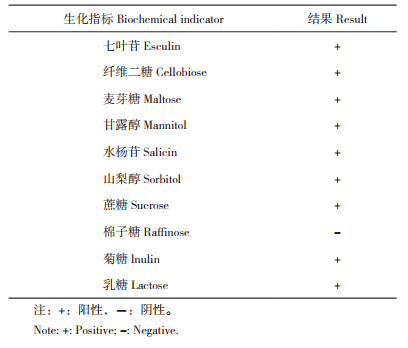
由表 3可知,菌株SZ07对万古霉素、头孢哌酮、氧氟沙星、氨苄西林、氯霉素、呋喃唑酮、头孢唑啉、多西环素、四环素、羧苄西林、头孢他啶敏感,对哌拉西林、多粘菌素B、青霉素、复方新诺明、苯唑西林等药物呈现出不同程度的耐药性。
2.6 菌株SZ07耐盐能力试验
由图 4可知,在不同的盐浓度条件下,菌株SZ07表现出不同的生长情况。NaCl浓度为0%~8% 时,菌株SZ07具有一定耐受能力,OD600值随着NaCl浓度的升高而有所降低。当NaCl浓度增加到8% 时,与低浓度相比,菌株的迟滞期更久,但仍然可生长。NaCl浓度为12%~16% 时,SZ07菌株生长繁殖受到抑制。NaCl浓度增加到12% 和16% 时,菌株基本不生长。
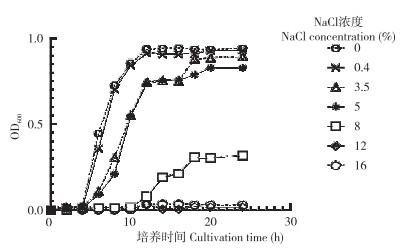
|
| 图 4 粪肠球菌SZ07耐盐能力测定结果 Fig. 4 Determination results of salt tolerance of strain SZ07 |
2.7 菌株SZ07毒力基因检测结果
对菌株SZ07进行8种主要毒力基因的PCR扩增,其中efaA、agg、as、fsrA共4种毒力基因(图 5)被检测到,而ace、esp、BlaTEM、cylA这4种毒力基因未被检测到。efaA、agg、as、fsrA与细菌的聚集物质、生物膜形成等功能有关,有助于其黏附在肠道上皮细胞,从而在宿主体内发挥作用。
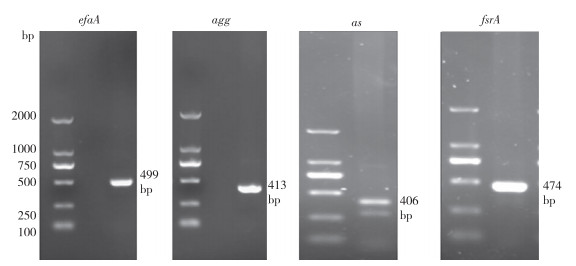
|
| 图 5 毒力基因PCR产物电泳结果 Fig. 5 Electrophoresis results of PCR products of virulence genes |
3 讨论
肠道菌群可通过调节免疫机制、保护肠道屏障等维持宿主体内稳态[26],还能促进机体消化食物、通过分泌次级代谢产物维持宿主健康[27]。肠道菌群与宿主之间相互依存和相互制约[28]。肠道菌群结构和功能失衡与多种疾病有关,因此肠道菌群紊乱可能影响宿主的免疫健康。水产养殖中长期使用抗生素会加速细菌耐药基因的传播[29],益生菌作为理想的抗生素替代品[30],被证明具有益生潜力。拌料饲喂益生菌可提高鱼体免疫力、预防疾病[31]。近年来越来越多学者研发应用于水产养殖业的潜在益生菌[32],比如,有研究发现饲喂枯草芽孢杆菌可显著增强卵形鲳鲹幼鱼的免疫应答和抗病性[4]。Doan等[33]研究表明饲喂植物乳杆菌可显著提升尼罗罗非鱼对无乳链球菌的抗病力。
常见的益生菌主要来自乳杆菌属(Lactobacillus spp.)、双歧杆菌属(Bifidobacterium spp.)、芽孢杆菌属(Bacillus spp.)和肠球菌属(Enterococcus spp.)[34]。其中水产养殖中常用的益生菌包括乳酸菌、硝化细菌、枯草芽孢杆菌、粪肠球菌等[35-37]。肠球菌最初被归类于肠道内革兰阳性球菌,后被划分为链球菌属,随着分子生物学的不断发展,1984年肠球菌从链球菌属中独立出来,正式归类于肠球菌属(当时由粪肠球菌和屎肠球菌2个菌种组成,后续又增加其他菌种)[38]。益生菌菌株一般具有耐受胃肠道环境、黏附性强、可抑制致病菌、安全性高的特性,但也会保留致病菌的一些毒力基因,比如efaA、agg、ace等,毒力基因表达的蛋白有助于粪肠球菌在肠道中定殖,从而发挥其潜在的益生作用[39]。本研究从粪肠球菌中检测出编码黏附相关蛋白的毒力基因,如as基因与细菌的聚集物质有关,可以增强粪肠球菌的黏附能力;fsrA基因与生物膜的形成有关;efaA、agg等基因有助于其黏附在肠道上皮细胞,从而在宿主体内发挥作用。黏附能力是筛选益生菌的重要标准之一,拥有良好的形成生物膜能力也可防止其他不良微生物在肠道定殖。肠球菌作为肠道中的正常菌群,对健康个体的风险性较小,因此在食品生产中通常被视为安全的菌群[40-41]。目前已有较多研究表明,从食品中分离到的粪肠球菌具有较强的抗炎和抗菌性,比如从突尼斯发酵乳中分离到的粪肠球菌能够产生抑制李斯特菌的细菌素[42]。调查发现,益生性肠球菌并不缺乏毒力因子或抗生素耐药性[41]。本研究中的分离菌株对万古霉素敏感,降低其传播抗生素耐药性的风险,但仍需对其潜在风险性进行详细研究。
粪肠球菌为革兰氏阳性菌,是鱼类肠道中的正常菌群,也是动物肠道中存在的天然益生菌[43]。粪肠球菌还可产生一系列抑菌物质,能产酸并消耗氧气,形成酸性的厌氧条件,抑制病原菌生长的同时促进乳酸菌生长[44]。粪肠球菌在维持宿主健康方面发挥多重作用,研究表明粪肠球菌可改善肠道健康、提高生长性能、调节免疫系统,增强鱼的抗病能力[45]。目前常用作水产动物饲料添加剂的益生菌来源于人类或陆生动物,益生菌的种属特异性导致此类非鱼源益生菌在水产环境中应用效果不佳。一些细菌只有与特定的宿主结合才能发挥良好的作用效果[46],与非鱼源宿主的益生菌相比,鱼源益生菌在同源宿主体内黏附性能等方面更具竞争力。海洋中存在大量益生菌,目前已从海洋动物肠道中分离得到多种乳酸菌[47]。从水生动物(原始宿主)肠道中分离筛选水产益生菌成为首选,其在水生环境中可发挥更理想的效果[48]。
4 结论本研究成功从卵形鲳鲹肠道中分离得到一株粪肠球菌SZ07,经生物学特性试验发现其具有较好的耐盐性和产酸能力。菌株SZ07对万古霉素、头孢哌酮、氧氟沙星等抗生素敏感;毒力基因鉴定结果表明,菌株SZ07含有efaA、agg、as、fsrA 4种与增强黏附能力相关的毒力基因,有助于其在肠道定殖。以上结果表明粪肠球菌SZ07具有用作饲料中益生菌添加剂的潜力,但其益生效果还需进一步研究。
| [1] |
LIU M J, GUO H Y, GAO J, ZHU K C, GUO L, LIU B S, ZHANG N, JIANG S G, ZHANG D C. Characteristics of microplastic pollution in golden pompano (Trachinotus ovatus) aquaculture areas and the relationship between colonized-microbiota on microplastics and intestinal microflora[J]. Science of the Total Environment, 2023, 856(2): 159-180. DOI:10.1016/j.scitotenv.2022.159180 |
| [2] |
LIU B, SAN L, GUO H, ZHU K, ZHANG N, YANG J, LIU B, HOU J, ZHANG D. Transcriptomic analysis reveals functional interaction of mRNA-lncRNA-miRNA in Trachinotus ovatus infected by Cryptocaryon irritans[J]. International Journal of Molecular Sciences, 2023, 24(21): 15886. DOI:10.3390/ijms242115886 |
| [3] |
LI P, ZHOU L, NI S, XU M, YU Y, CAI J, WEI S, QIN Q. Establishment and characterization of a novel cell line from the brain of golden pompano (Trachinotus ovatus)[J]. In Vitro Cellular & Developmental Biology–Animal, 2016, 52(4): 410-418. DOI:10.1007/s11626-015-9988-6 |
| [4] |
ZHANG Q, YU H, TONG T, TONG W, DONG L, XU M, WANG Z. Dietary supplementation of Bacillus subtilis and fructooligosaccharide enhance the growth, non-specific immunity of juvenile ovate pompano, Trachinotus ovatus and its disease resistance against Vibrio vulnificus[J]. Fish & Shellfish Immunology, 2014, 38(1): 7-14. DOI:10.1016/j.fsi.2014.02.008 |
| [5] |
陈建国, 黄荣静. 危害鱼类的几种常见纤毛虫[J]. 河南水产, 2007(2): 29-30. CHEN J G, HUANG R J. Several common ciliates that harm to fish[J]. Henan Fisheries, 2007(2): 29-30. |
| [6] |
熊向英, 徐力文, 董兰芳, 王志成, 梁志辉, 蒋伟添, 黄国强. 网箱养殖卵形鲳鲹鱼体寄生虫初步调查[J]. 广西科学院学报, 2015, 31(4): 281-285. DOI:10.13657/j.cnki.gxkxyxb.20151126.013 XIONG X Y, XU L W, DONG L F, WANG Z C, LIANG Z H, JIANG W T, HUANG G Q. Study on the parasites from Trachinotus ovatus in marine cage culture[J]. Journal of Guangxi Academy of Sciences, 2015, 31(4): 281-285. DOI:10.13657/j.cnki.gxkxyxb.20151126.013 |
| [7] |
李安. 患刺激隐核虫病卵形鲳鲹的继发细菌疾病研究[D]. 海口: 海南大学, 2020. DOI: 10.27073/d.cnki.ghadu.2020.001411. LI A. Study on secondary l diseases of Trachinotus ovatus with cryptocaryoniasis[D]. Haikou: Hainan University, 2020. DOI: 10.27073/d.cnki.ghadu.2020.001411. |
| [8] |
LIN S, MAO S, GUAN Y, LIN X, LUO L. Dietary administration of chitooligosaccharides to enhance growth, innate immune response and disease resistance of Trachinotus ovatus[J]. Fish & Shellfish Immunology, 2012, 32(5): 909-913. DOI:10.1016/j.fsi.2012.02.019 |
| [9] |
ZHOU C, LIN H, GE X, NIU J, WANG J, WANG Y, CHEN L, HUANG Z, YU W, TAN X. The effects of dietary soybean isoflavones on growth, innate immune responses, hepatic antioxidant abilities and disease resistance of juvenile golden pompano Trachinotus ovatus[J]. Fish & Shellfish Immunology, 2015, 43(1): 158-166. DOI:10.1016/j.fsi.2014.12.014 |
| [10] |
DO HUU H, SANG H M, THANH THUY N T. Dietary β-glucan improved growth performance, Vibrio counts, haematological parameters and stress resistance of pompano fish, Trachinotus ovatus Linnaeus[J]. Fish & Shellfish Immunology, 2016, 54: 402-410. DOI:10.1016/j.fsi.2016.03.161 |
| [11] |
XIONG X, CHEN R, LAI J. Comparative genomics analysis of Streptococcus iniae isolated from Trachinotus ovatus: Novel insight into antimicrobial resistance and virulence differentiation[J]. BMC Genomics, 2023, 24(1): 775. DOI:10.1186/s12864-023-09882-5 |
| [12] |
QIU W, LIU T, LIU X, CHEN H, LUO S, CHEN Q, MAGNUSON J T, ZHENG C, XU E G, SCHLENK D. Enrofloxacin induces intestinal microbiota-mediated immunosuppression in Zebrafish[J]. Environmental Science & Technology, 2022, 56(12): 8428-8437. DOI:10.1021/acs.est.1c08712 |
| [13] |
凡中坤, 朱凯, 沈斌, 徐建雄, 张洪才. 复合益生菌在畜禽养殖中应用的研究进展[J]. 饲料工业, 2022, 43(19): 18-22. DOI:10.13302/j.cnki.fi.2022.19.004 FAN Z K, ZHU K, SHEN B, XU J X, ZHANG H C. Recent advances of the application of compound probiotics in livestock breeding[J]. Feed Industry, 2022, 43(19): 18-22. DOI:10.13302/j.cnki.fi.2022.19.004 |
| [14] |
KUEBUTORNYE F K A, ABARIKE E D, LU Y. A review on the application of Bacillus as probiotics in aquaculture[J]. Fish & Shellfish Immunology, 2019, 87: 820-828. DOI:10.1016/j.fsi.2019.02.010 |
| [15] |
PEREIRA W A, MENDONÇA C M N, URQUIZA A V, MARTEINSSON V Þ, LEBLANC J G, COTTER P D, VILLALOBOS E F, ROMERO J, OLIVEIRA R P S. Use of probiotic bacteria and bacteriocins as an alternative to antibiotics in aquaculture[J]. Microorganisms, 2022, 10(9): 1705. |
| [16] |
陈丽婷, 吴剑峰, 赵玉兵, 严欣, 肖俊, 罗永巨, 项桂德, 梁军能. 零换水条件下复合益生菌对罗非鱼生长性能、肌肉品质及养殖水体环境的影响[J]. 广东农业科学, 2022, 49(4): 123-134. DOI:10.16768/j.issn.1004-874X.2022.04.015 CHEN L T, WU J F, ZHAO Y B, YAN X, XIAO J, LUO Y J, XIANG G D, LIANG J N. Effects of compound probiotics on growth performance, muscle quality and aquaculture water of tilapia under zero-water exchange condition[J]. Guangdong Agricultural Sciences, 2022, 49(4): 123-134. DOI:10.16768/j.issn.1004-874X.2022.04.015 |
| [17] |
REDA R M, SELIM K M, EL-SAYED H M, EL-HADY M A. In vitro selection and identification of potential probiotics isolated from the gastrointestinal tract of Nile tilapia, Oreochromis niloticus[J]. Probiotics and Antimicrobial Proteins, 2018, 10(4): 692-703. DOI:10.1007/s12602-017-9314-6 |
| [18] |
LIM Y J, HAM H, LEE M H, PARK D S, ROH E, PARK D H, LEE Y H. First report of fire blight on chinese hawthorn (Crataegus pinnatifida Bunge) caused by Erwinia amylovora in Korea[J]. Plant Disease, 2023(6): 13-17. DOI:10.1094/PDIS-04-23-0703-PDN |
| [19] |
World Health Organization. FAO/WHO working group report on drafting guidelines for the evaluation of probiotics in food[EB/OL]. Ontario, Canada: World Health Organization. http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. 2002.
|
| [20] |
IWERIEBOR B C, OBI L C, OKOH A I. Virulence and antimicrobial resistance factors of Enterococcus spp. isolated from fecal samples from piggery farms in Eastern Cape, South Africa[J]. BMC Microbiology, 2015, 15: 136. DOI:10.1186/s12866-015-0468-7 |
| [21] |
WILLEMS R J, HOMAN W, TOP J, VAN SANTEN-VERHEUVEL M, TRIBE D, MANZIOROS X, GAILLARD C, VANDENBROUCKE-GRAULS C M, MASCINI E M, VAN KREGTEN E, VAN EMBDEN J D, BONTEN M J. Variant esp gene as a marker of a distinct genetic lineage of vancomycin-resistant Enterococcus faecium spreading in hospitals[J]. The Lancet, 2001, 357(9259): 853-855. DOI:10.1016/S0140-6736(00)04205-7 |
| [22] |
RANA M L, FIRDOUS Z, FERDOUS F B, ULLAH M A, SIDDIQUE M P, RAHMAN M T. Antimicrobial resistance, biofilm formation, and virulence determinants in Enterococcus faecalis isolated from cultured and wild fish[J]. Antibiotics (Basel), 2023, 12(9): 1375. DOI:10.3390/antibiotics12091375 |
| [23] |
STRATEVA T, ATANASOVA D, SAVOV E, PETROVA G, MITOV I. Incidence of virulence determinants in clinical Enterococcus faecalis and Enterococcus faecium isolates collected in Bulgaria[J]. The Brazilian Journal of Infectious Diseases, 2016, 20(2): 127-133. DOI:10.1016/j.bjid.2015.11.011 |
| [24] |
王亚宾, 陈丽颖, 张红英, 刘磊, 程金平, 崔保安. 感染仔猪粪肠球菌不同分离株的鉴定及毒力基因检测[J]. 中国兽医学报, 2010, 30(5): 615-619. DOI:10.16303/j.cnki.1005-4545.2010.05.018 WANG Y B, CHEN L Y, ZHANG H Y, LIU L, CHENG J P, CUI B A. Identification and determination of virulence gene for different isolates of E. faecalis originated from infective piglet[J]. Chinese Journal of Veterinary Science, 2010, 30(5): 615-619. DOI:10.16303/j.cnki.1005-4545.2010.05.018 |
| [25] |
王佰涛, 王秋菊, 雷高, 杨文玲, 权淑静, 刘德海. 1株高产乳酸粪肠球菌的筛选、鉴定及益生性能的研究[J]. 饲料研究, 2023, 46(1): 95-99. DOI:10.13557/j.cnki.issn1002-2813.2023.01.020 WANG B T, WANG Q J, LEI G, YANG W L, QUAN S J, LIU D H. Screening, identification and probiotic property of a Enterococcus faecalis strain with high lactic acid production[J]. Feed Research, 2023, 46(1): 95-99. DOI:10.13557/j.cnki.issn1002-2813.2023.01.020 |
| [26] |
YOU C, CHEN B, WANG M, WANG S, ZHANG M, SUN Z, JUVENTUS A J, MA H, LI Y. Effects of dietary lipid sources on the intestinal microbiome and health of golden pompano (Trachinotus ovatus)[J]. Fish & Shellfish Immunology, 2019, 89: 187-197. DOI:10.1016/j.fsi.2019.03.060 |
| [27] |
PURI P, SHARMA J G, SINGH R. Biotherapeutic microbial supplementation for ameliorating fish health: Developing trends in probiotics, prebiotics, and synbiotics use in finfish aquaculture[J]. Animal Health Research Reviews, 2022, 23(2): 113-135. DOI:10.1017/S1466252321000165 |
| [28] |
PARK J S, GAZZANIGA F S, KASPER D L, SHARPE A H. Microbiota-dependent regulation of costimulatory and coinhibitory pathways via innate immune sensors and implications for immunotherapy[J]. Experimental & Molecular Medicine, 2023, 55(9): 1913-1921. DOI:10.1038/s12276-023-01075-0 |
| [29] |
卢春兰, 王蓓. 壳寡糖和几丁寡糖的制备方法及其在水产上的应用[J]. 广东农业科学, 2023, 50(2): 136-146. DOI:10.16768/j.issn.1004-874X.2023.02.015 LU C L, WANG B. Preparation method of chitosan oligosaccharide and chitin oligosaccharide and their application in aquaculture[J]. Guangdong Agricultural Sciences, 2023, 50(2): 136-146. DOI:10.16768/j.issn.1004-874X.2023.02.015 |
| [30] |
伍楚妍, 黄晓冰, 刘少君, 李洋, 谢为天, 徐春厚. 海洋源芽孢杆菌的分离鉴定及其消化酶代谢产物的测定[J]. 广东农业科学, 2021, 48(7): 137-144. DOI:10.16768/j.issn.1004-874X.2021.07.017 WU C Y, HUANG X B, LIU S J, LI Y, XIE W T, XU C H. Isolation and identification of marine Bacillus and determination of its digestive enzyme metabolites[J]. Guangdong Agricultural Sciences, 2021, 48(7): 137-14. DOI:10.16768/j.issn.1004-874X.2021.07.017 |
| [31] |
DE MARCO G, CAPPELLO T, MAISANO M. Histomorphological changes in fish gut in response to prebiotics and probiotics treatment to improve their health status: A review[J]. Animals (Basel), 2023, 13(18): 28-36. DOI:10.3390/ani13182860 |
| [32] |
BANERJEE G, RAY A K. The advancement of probiotics research and its application in fish farming industries[J]. Research in Veterinary Science, 2017, 115: 66-77. DOI:10.1016/j.rvsc.2017.01.016 |
| [33] |
VAN DOAN H, HOSEINIFAR S H, TAPINGKAE W, SEEL-AUDOM M, JATURASITHA S, DAWOOD M A O, WONGMANEEPRATEEP S, THU T T N, ESTEBAN M Á. Boosted growth performance, mucosal and serum immunity, and disease resistance Nile tilapia (Oreochromis niloticus) fingerlings using corncob-derived xylooligosaccharide and Lactobacillus plantarum CR1T5[J]. Probiotics and Antimicrobial Proteins, 2020, 12(2): 400-411. DOI:10.1007/s12602-019-09554-5 |
| [34] |
王怡然, 丁丽敏, 赵丽红. 益生菌对宠物健康的调控作用及机制研究进展[J]. 中国畜牧杂志, 2024, 60(3): 1-8. DOI:10.19556/j.0258-7033.20230420-05 WANG Y R, DING L M, ZHAO L H. Advance in the function and mechanism of probiotics in regulating pet health[J]. Chinese Journal of Animal Science, 2024, 60(3): 1-8. DOI:10.19556/j.0258-7033.20230420-05 |
| [35] |
李长玲, 黄翔鹄, 李瑞伟, 区启鸿. 硝化细菌对罗非鱼苗培育环境及抗病力的影响[J]. 广东海洋大学学报, 2008, 28(6): 41-45. DOI:10.3969/j.issn.1673-9159.2008.06.009 LI C L, HUANG X H, LI R W, OU Q H. Effects of nitrifying bacteria on culture environment and anti-disease ability of larval tilapia[J]. Journal of Guangdong Ocean University, 2008, 28(6): 41-45. DOI:10.3969/j.issn.1673-9159.2008.06.009 |
| [36] |
蒋鑫涛, 陈有铭, 黄鉴鹏, 欧光海, 温震威, 李豫, 马骞, 陈刚. 复合益生菌对杂交石斑鱼生长性能、抗氧化能力和肠道健康的影响[J]. 广东海洋大学学报, 2023, 43(5): 81-91. DOI:10.3969/j.issn.1673-9159.2023.05.011 JIANG X T, CHEN Y M, HUANG J P, OU G H, WEN Z W, LI Y, MA Q, CHEN G. Effects of compound probiotics on growth performance, antioxidant capacity and intestinal health of hybrid grouper (Epinephelus fuscogutatus ♀×Epinephelus polyphekadion ♂)[J]. Journal of Guangdong Ocean University, 2023, 43(5): 81-91. DOI:10.3969/j.issn.1673-9159.2023.05.011 |
| [37] |
艾影, 王维政, 陈刚, 张健东, 黄建盛, 潘传豪, 施钢, 谢瑞涛, 周晖, 汤保贵. 2种乳酸菌对军曹鱼幼鱼生长及消化酶、免疫酶活性的影响[J]. 广东海洋大学学报, 2020, 40(5): 112-117. DOI:10.3969/j.issn.1673-9159.2020.05.014 AI Y, WANG W Z, CHEN G, ZHANG J D, HUANG J S, PAN C H, SHI G, XIE R T, ZHOU H, TANG B G. Effects of two lactic acid bacteria on growth performance and activities of digestive and non-specific immune enzymes of juvenile cobia (Rachycentron canadum)[J]. Journal of Guangdong Ocean University, 2020, 40(5): 112-117. DOI:10.3969/j.issn.1673-9159.2020.05.014 |
| [38] |
李金钟. 肠球菌分类与鉴定新进展[J]. 临床检验杂志, 2006(3): 228-230. DOI:10.13602/j.cnki.jcls.2006.03.040 LI J Z. New progress in classification and identification of Enterococcus[J]. Chinese Journal of Clinical Laboratory Science, 2006(3): 228-230. DOI:10.13602/j.cnki.jcls.2006.03.040 |
| [39] |
ALKALBANI N S, TURNER M S, AYYASH M M. Isolation, identification, and potential probiotic characterization of isolated lactic acid bacteria and in vitro investigation of the cytotoxicity, antioxidant, and antidiabetic activities in fermented sausage[J]. Microbial Cell Factories, 2019, 18(1): 188. DOI:10.1186/s12934-019-1239-1 |
| [40] |
AKTER T, HAQUE M N, EHSAN R, PAUL S I, FOYSAL M J, TAY A C Y, ISLAM M T, RAHMAN M M. Virulence and antibiotic-resistance genes in Enterococcus faecalis associated with streptococcosis disease in fish[J]. Scientific Reports, 2023, 13(1): 1551. DOI:10.1038/s41598-022-25968-8 |
| [41] |
CEBRIÁN R, BAÑOS A, VALDIVIA E, PÉREZ-PULIDO R, MARTÍNEZ-BUENO M, MAQUEDA M. Characterization of functional, safety, and probiotic properties of Enterococcus faecalis UGRA10, a new AS-48-producer strain[J]. Food Microbiology, 2012, 30(1): 59-67. DOI:10.1016/j.fm.2011.12.002 |
| [42] |
HANCHI H, HAMMAMI R, KOURDA R, HAMIDA J B, FLISS I. Bacteriocinogenic properties and in vitro probiotic potential of enterococci from Tunisian dairy products[J]. Archives of Microbiology, 2014, 196(5): 331-344. DOI:10.1007/s00203-014-0978-y |
| [43] |
PAN X, KONG R, LIU Q, JIA Z, BAI B, CHEN H, ZHI W, WANG B, MA C, MA D. Probiotic Enterococcus faecalis surface-delivering key domain of EtMIC3 proteins: Immunoprotective efficacies against Eimeria tenella infection in chickens[J]. Microbiology Spectrum, 2023, 11(6): e02455. DOI:10.1128/spectrum.02455-23 |
| [44] |
缑敬轩, 吕嘉枥, 张智维, 宋宏新. 泡菜中益生性乳酸菌的筛选和鉴定[J]. 中国酿造, 2008, 27(11): 22-24. GOU J X, LYU J L, ZHANG Z W, SONG H X. Isolation and identification of probiotic lactic acid bacteria starter from pickles[J]. China Brewing, 2008, 27(11): 22-24. |
| [45] |
DING X Y, WEI C Y, LIU Z Y, YANG H L, HAN F, SUN Y Z. Autochthonous Bacillus subtilis and Enterococcus faecalis improved liver health, immune response, mucosal microbiota and red-head disease resistance of yellow drum (Nibea albiflora)[J]. Fish & Shellfish Immunology, 2023, 134: 108575. DOI:10.1016/j.fsi.2023.108575 |
| [46] |
王思盼, 陈柏安, 黄永健, 林坤, 崔建军, 刘东超. 长茎葡萄蕨藻益藻细菌筛选及性能评价[J]. 广东海洋大学学报, 2023, 43(4): 84-92. DOI:10.3969/j.issn.1673-9159.2023.04.011 WANG S P, CHEN B A, HUANG Y J, LIN K, CUI J J, LIU D C. Screening and performance evaluation of beneficial bacteria from Caulerpa lentillifera[J]. Journal of Guangdong Ocean University, 2023, 43(4): 84-92. DOI:10.3969/j.issn.1673-9159.2023.04.011 |
| [47] |
唐慧芳, 张瀛, 房志家, 孙力军, 刘颖. 海洋动物肠道中抗氧化活性乳酸菌的分离及多样性分析[J]. 广东海洋大学学报, 2019, 39(2): 104-110. DOI:10.3969/j.issn.1673-9159.2019.02.013 TANG H F, ZHANG Y, FANG Z J, SUN L J, LIU Y. Screening and diversity analysis of marine lactic acid bacteria with antioxidant activity[J]. Journal of Guangdong Ocean University, 2019, 39(2): 104-110. DOI:10.3969/j.issn.1673-9159.2019.02.013 |
| [48] |
TIMMERMAN H M, VELDMAN A, VAN DEN ELSEN E, ROMBOUTS F M, BEYNEN A C. Mortality and growth performance of broilers given drinking water supplemented with chicken-specific probiotics[J]. Poultry Science, 2006, 85(8): 1383-1388. DOI:10.1093/ps/85.8.1383 |
(责任编辑 陈丽娥)
 2024, Vol. 51
2024, Vol. 51