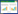文章信息
基金项目
- 国家自然科学基金(32160784);广东省农业动物基因组学与分子育种重点实验室开放课题;江西省自然科学基金(20171BAB204013)
作者简介
- 张文武(1985—),男,博士,讲师,研究方向为鸡遗传育种与繁殖,E-mail:125714992@qq.com.
通讯作者
- 许继国(1982—),男,博士,副教授,研究方向为家禽育种,E-mail:3425614@qq.com.
文章历史
- 收稿日期:2022-09-15
2. 南昌师范学院生物技术研究所 / 江西地方鸡种遗传改良重点实验室,江西 南昌 330029
2. Institute of Biological Technology, Nanchang Normal University / Jiangxi Key Laboratory of Genetic Improvement of Indigenous Chicken Breeds, Nanchang 330029, China
【研究意义】了解物种内和物种间表型多样性的遗传基础是现代生物学研究的重大挑战之一。家鸡经过数千年的选择性繁殖聚集了丰富的表型变异,是研究和理解表型多样性遗传基础的重要研究材料。【前人研究进展】鸡冠作为易见的装饰性状,无论在为人类提供肉蛋的商业化品种还是作为观赏用品种上,均受到强烈的人工选择[1]。作为鸡的第二性征,鸡冠与性成熟之间的关系已经得到很好的研究。科学家还发现鸡冠与交配决策、社会等级、体温调节、生殖能力和骨量有重要关系[2-7]。研究表明,多因子调控鸡冠不同形态。通过转录组测序技术已鉴定到与鸡冠发育相关的候选基因有BMP2基因和CHADL基因[8-9]。三叉冠是单冠后端两侧分裂出肉质隆起,形态像叉状(图 1)。Pearl等[10]最早提出三叉冠性状,认为三叉冠是纯单冠和纯豆冠的中间态。有学者通过白来航鸡的杂交试验发现两个常染色体上有两对等位基因控制单冠和分叉,但试验群体不大,其结论仍有待进一步验证[11]。有学者通过杂交试验发现三叉冠基因型为Sss1s1、SSs1s1、ssS1S1、ssS1s1和sss1s1,非三叉基因型为SSS1S1、SsS1S1、SSS1s1和SsS1s1最符合交配试验后代的分布比例,但该假设未能很好解析左叉和右叉等冠型的遗传规律,推测三叉冠是多基因遗传控制的性状[12]。目前对其他冠型方面的研究成果很多,有研究者发现细齿冠宁都黄公鸡的AR与VIPR1蛋白免疫组织化学阳性细胞比率显著低于其他冠型个体,倒冠个体的AR与VIPR1蛋白的免疫组织化学阳性细胞比率均显著高于其他冠型个体,并且在鸡冠窦状血管网内皮细胞及生发层细胞中具有较高免疫组织化学阳性反应[13]。研究人员发现玫瑰冠纯合子的公鸡个体生育能力较低[14]。携带豆冠等位基因的个体其肉垂也比较小且龙骨皮肤变厚[15]。由于进化保守区的重复导致SOX5基因的异位表达,而异位表达的SOX5基因又改变了SSH基因的表达,从而导致鸡冠异常发育[16]。Somes[17]认为该基因座位还存在一种控制蝴蝶冠的等位基因,其显隐性位于V形冠等位基因(DV)和双冠等位基因(DC)之间。基因组重复与鸡胚鸡冠和两种双鸡冠表型的异位异胚层表达有关[18]。

|
| 图 1 白耳黄鸡三叉冠性状 Fig. 1 Trigeminal comb traits of white-ear yellow chickens |
【本研究切入点】随着近几年分子生物学技术手段的进步,玫瑰冠、豆冠和双冠等主要冠型的致因突变已经鉴定且性状形成机制也已被初步阐明,但目前尚无有关三叉冠性状的分子遗传学研究。【拟解决的关键问题】生产中发现,白耳黄鸡群体有约5% 的个体为三叉冠。本研究以白耳黄鸡为研究材料,采用杂交实验、受精率实验和全基因组关联分析(Genome-wide Association Analysis, GWAS)对白耳黄鸡三叉冠性状进行分析,以期为三叉冠的进一步研究提供参考。
1 材料与方法 1.1 试验材料供试白耳黄鸡来自江西省上饶市广丰区白耳黄鸡原种场。供试白耳黄鸡的饲养管理流程按原种场管理;饲料营养方面,育雏阶段(1~30日龄)饲喂正大慢速品种小鸡料,育成阶段(30~170日龄)饲喂正大育成料。
1.2 试验方法1.2.1 正反交试验和受精率试验 以60只120日龄的白耳黄鸡进行正反交试验,15只三叉冠公鸡与15只单冠母鸡交配,以及15只单冠公鸡与15只三叉冠母鸡交配。以120只120日龄的白耳黄鸡进行受精率试验,30只单冠公鸡和30只三叉冠公鸡分别与30只单冠母鸡交配。
1.2.2 GWAS分析 对82只150日龄的白耳黄公鸡(23只三叉冠、23只单冠)于翅静脉采血各5 mL,用EDTA抗凝处理,提取基因组DNA用于GWAS分析。基因分型采用Illumina平台的鸡京芯一号60 k芯片。数据质控使用PLINK 1.90软件。基因型质控过程将删除达到以下标准的个体或位点:分型缺失数 > 10% 的位点、最小等位基因频率 < 0.01的位点和哈代-温伯格检验P值< 1×10-4的位点。
在GWAS分析中,试验结果的假阳性部分来自群体分层[19]。主成分分析(PCA)方法能用于遗传学的聚类分析,根据遗传差异可将个体分为不同亚组。本研究采用PCA方法进行群体分层分析,使用GCTA软件对有效SNP进行PCA,获得基因组数据特征值和特征向量,并用R程序作PCA图。GWAS分析采用混合线性模型GEMMA。采用Bonferroni多重检验方法确定关联显性阈值,阈值为1/有效SNP位点数量。
在NCBI网站上下载对应物种的参考基因组信息(https://www.ncbi.nlm.nih.gov/genome/111?genome_assembly_id=1543395),使用ANNOVAR软件将显著SNP注释到其对应的基因上,并结合文献注释候选基因的功能
2 结果与分析 2.1 白耳黄鸡三叉冠表型遗传方式用三叉冠白耳黄鸡个体与单冠白耳黄鸡个体进行正反交试验,结果(表 1)显示后代全为单冠。在遗传杂交试验中通常采用正交和反交来判断某性状的基因是位于常染色体上还是性染色体上。根据后代全为单冠初步推测,白耳黄鸡三叉冠为常染色体遗传。
2.2 单冠和三叉冠白耳黄鸡的种蛋受精率比较
比较30只单冠公鸡和30只三叉冠公鸡与配单冠母鸡的受精能力,结果显示,与配单冠群体的种蛋受精率为94.9%(258/272),与配三叉冠群体的种蛋受精率为92.7%(241/260),差异不显著。
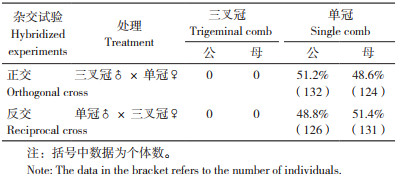
2.3 白耳黄鸡群体有效SNP数和群体结构分析GWAS分析首先要对基因型及表型进行一定标准的质控,以排除异常基因型和异常表型对分析结果产生的影响。经质控白耳黄鸡群体得到55 023个有效SNP。通过对标记矩阵进行PCA分析,提取解释方差最大的前几位PCA,绘制对应散点图可反映群体结构。从图 2可以看出,白耳黄鸡群体的点相对集中,表明白耳黄鸡群体内不存在明显的群体分层,可以进行后续分析。
|
| 图 2 白耳黄鸡群体结构分析 Fig. 2 Population structure analysis of white-ear yellow chickens |
2.4 白耳黄鸡三叉冠显著关联SNP位点分析
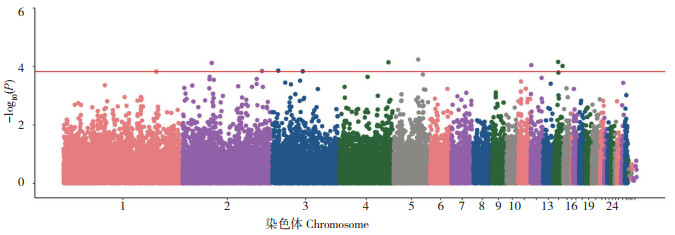
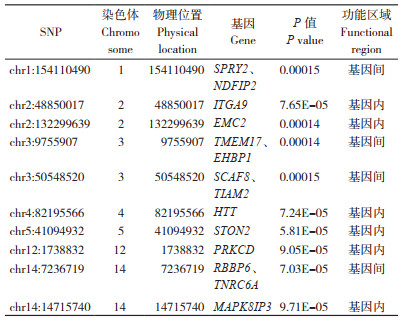
图 3和图 4分别是对白耳黄鸡三叉冠GWAS分析所得结果的QQ图和曼哈顿图。QQ图中X轴和Y轴分别为期望的P值和实际的P值,两者一致时,由随机漂变导致;两者不一致时,存在真实变异,对应的点与表型相关。在曼哈顿图中每个基因组SNP位点沿染色体序列排列,Y轴的P值可理解为每个SNP与表型的关联程度。结果(表 2)显示,与白耳黄鸡三叉冠显著关联SNP位点共有10个,其中有1个SNP位于第1号染色体,2个SNP位于第2号染色体,2个SNP位于第3号染色体,1个SNP位于第4号染色体,1个SNP位于第5号染色体,1个SNP位于第12号染色体,2个SNP位于第14号染色体。这10个SNP分别邻近或座落于SPRY2、NDFIP2、ITGA9、EMC2、TMEM17、EHBP1、SCAF8、TIAM2、HTT、STON2、PRKCD、RBBP6、TNRC6A、MAPK8IP3基因上。

|
| 图 3 白耳黄鸡三叉冠关联分析QQ图 Fig. 3 QQ plot of association analysis of white-ear yellow chicken with trigeminal comb |
|
| 图 4 白耳黄鸡三叉冠关联分析曼哈顿图 Fig. 4 Manhattan diagram of association analysis of white-ear yellow chicken with trigeminal comb |
|
3 讨论 3.1 白耳黄鸡三叉冠遗传方式
在遗传杂交试验中通常采用正交和反交来判断某性状的基因位于常染色体上还是性染色体上。有学者通过白色来航鸡的杂交试验发现两个常染色体上有两对等位基因控制单冠和分叉冠[11]。有学者通过杂交试验发现三叉冠基因型是常染色体多基因遗传控制的性状[12]。本研究发现白耳黄鸡三叉冠呈现常染色体遗传,这与前人研究结果相一致。
3.2 白耳黄鸡三叉冠与受精率的关系有研究报道,玫瑰冠与豆冠、单冠的精子活率和受精率差异不显著,但玫瑰冠与单冠在精子密度和受精蛋孵化率上显著高于豆冠[20]。有学者研究玫瑰冠个体的精液质量与不同基因型个体的生育能力和生育持续时间,发现精子活力缺陷是导致低生育能力以及生育时间短的原因[21]。但还未有研究三叉冠与受精率关系的报道,我们发现与配三叉冠公鸡的母鸡群体的种蛋受精率低于与配单冠公鸡个体,但差异不显著,具体原因还需要进一步研究。
3.3 白耳黄鸡三叉冠的GWAS分析在鸡生产中,GWAS被用来鉴定与生产相关性状的候选基因。本研究对白耳黄鸡三叉冠进行GWAS分析,检测到10个显著SNP,分别邻近或座落于SPRY2、NDFIP2、ITGA9、EMC2、TMEM17、EHBP1、SCAF8、TIAM2、HTT、STON2、PRKCD、RBBP6、TNRC6A和MAPK8IP3基因上。但根据前人研究结果[22-36],这些基因并非与白耳黄鸡三叉冠形成直接相关的候选基因。
4 结论本研究对白耳黄鸡三叉冠性状进行分析,结果显示,白耳黄鸡三叉冠呈现常染色体遗传;GWAS分析发现10个与白耳黄鸡三叉冠显著关联的SNP,分别邻近或座落于SPRY2、NDFIP2、ITGA9、EMC2、TMEM17、EHBP1、SCAF8、TIAM2、HTT、STON2、PRKCD、RBBP6、TNRC6A、MAPK8IP3基因上,但这些基因并非与白耳黄鸡三叉冠形成直接相关的候选基因,这些基因是否参与三叉冠的形成需要进一步证实。
| [1] |
许继国, 高鑫凤, 陈杰, 叶峭, 聂庆华, 张细权. 鸡冠常见冠型及其遗传基础研究进展[J]. 广东农业科学, 2016, 43(3): 162-166, 193. DOI:10.16768/j.issn.1004-874X.2016.03.032 XU J G, GAO X F, CHEN J, YE Q, NIE Q H, ZHANG X Q. Introduction of different combs and advances in their genetic basis[J]. Guangdong Agricultural Sciences, 2016, 43(3): 162-166, 193. DOI:10.16768/j.issn.1004-874X.2016.03.032 |
| [2] |
马猛, 王克华, 曲亮, 窦套存, 沈曼曼, 王星果, 郭军. 鸡72周龄产蛋性状与鸡冠性状典型相关分析[J]. 广东农业科学, 2019, 46(8): 118-122. DOI:10.16768/j.issn.1004-874X.2019.08.016 MA M, WANG K H, QU L, DOU T C, SHEN M M, WANG X G, GUO J. Analysis on canonical correlation between egg production and comb traits of 72-week-old chicken[J]. Guangdong Agricultural S cience s, 2019, 46(8): 118-122. DOI:10.16768/j.issn.1004-874X.2019.08.016 |
| [3] |
徐海平, 曾华, 周敏, 方梅霞, 沈栩, 张细权. 下丘脑-垂体-性腺轴上基因多态性与鸡冠高和体重的相关性分析[J]. 广东农业科学, 2011, 38(8): 4-7. DOI:10.16768/j.issn.1004-874X.2011.08.073 XU H P, ZENG H, ZHOU M, FANG M X, SHEN X, ZHANG X Q. Association of 10 polymorphisms in the candidate genes with chicken comb height and body weight traits[J]. Guangdong Agricultural Sciences, 2011, 38(8): 4-7. DOI:10.16768/j.issn.1004-874X.2011.08.073 |
| [4] |
WAN Y, WANG Z, GUO X, MA C D, FANG Q, GENG Z Y, CHEN X Y, JIANG R S. Phenotypic characteristics of upright and pendulous comb among chicken breeds and association with growth rate and egg production[J]. Animal Science Journal, 2018, 89(1): 250-256. DOI:10.1111/asj.12922 |
| [5] |
LIU Y, TU Y, ZHANG M, JI G G, WANG K, SHAN Y J, JU X J, ZHANG D, SHU J T, ZOU J M. Identification of molecular pathways and candidate genes associated with cocks' comb size trait by genome-wide transcriptome analysis[J]. Scientifi c Reports, 2018, 8(1): 2015. DOI:10.1038/s41598-018-20373-6 |
| [6] |
WANG Y, LI J, FENG C, ZHAO Y, HU X, LI N. Transcriptome analysis of comb and testis from Rose-comb Silky chicken (R1/R1) and Beijing Fatty wild type chicken (r/r)[J]. Poultry Science, 2017, 96(6): 1866-1873. DOI:10.3382/ps/pew447 |
| [7] |
ŁUKAZEWICZ E, LASON M, KOWALCZYK A, BEDNARCZYK M. Secondary sexual traits and semen characteristic of chicken germline chimeras[J]. Reproduction in Domestic Animals, 2018, 53(4): 859-863. DOI:10.1111/rda.13176 |
| [8] |
王琨, 刘一帆, 章明, 屠云洁, 姬改革, 单艳菊, 巨晓军, 束婧婷, 虞徳兵. 公鸡鸡冠发育相关候选基因的表达分析[J]. 中国家禽, 2017, 39(22): 5-9. DOI:10.16372/j.issn.1004-6364.2017.22.002 WANG K, LIU Y F, ZHANG M, TU Y J, JI G G, SHAN Y J, JU X J, SHU J T, YU D B. Expression of several candidate genes for comb development in cock[J]. China Poultry, 2017, 39(22): 5-9. DOI:10.16372/j.issn.1004-6364.2017.22.002 |
| [9] |
王琨, 刘一帆, 章明, 邹剑敏, 屠云洁, 姬改革, 巨晓军, 束婧婷, 虞徳兵. 不同周龄公鸡鸡冠组织学观察和BMP2、CHADL基因的表达分析[J]. 畜牧与兽医, 2018, 50(6): 7-11. WANG K, LIU Y F, ZHANG M, ZOU J M, TU Y J, JI G G, JU X J, SHU J T, YU D B. Histological observation and analysis of BMP2 and CHADL expression in the combs of different week-old cocks[J]. Animal Husbandry & Veterinary Medicine, 2018, 50(6): 7-11. |
| [10] |
PEARL R, PEARL M D. Data on variation in the comb of the domestic fowl[J]. Biometrika, 1909, 6(4): 420-432. DOI:10.1093/biomet/6.4.420 |
| [11] |
ASMUNDSON V S. Inheritance of side sprigs: data on the inheritance of side sprigs on the combs of single comb White Leghorns[J]. Journal of Heredity, 1926, 17(8): 281-284. DOI:10.1093/oxfordjournals.jhered.a102732 |
| [12] |
韦金兑. 清远麻鸡、广西麻鸡冠型生产性能及遗传规律分析[D]. 佛山: 佛山科学技术学院, 2020. WEI J D. Genetics and production performance of comb types in Qingyuan Partridge Chickens and Guangxi Partridge Chickens[D]. Foshan: Foshan University, 2020. |
| [13] |
贡继尚, 周敏. AR与VIPR1蛋白在不同表型鸡冠的免疫组织化学与比较组织学研究[J]. 黑龙江畜牧兽医, 2022(3): 15-18, 129. DOI:10.13881/J.CNKI.HLJXMSY.2021.04.0041 GONG J S, ZHOU M. Immunohistochemical and comparative histological study of AR and VIPR1 protein in cock combs with different phenotypes[J]. Heilong jiang Animal Science and Veterinary Medicine, 2022(3): 15-18, 129. DOI:10.13881/J.CNKI.HLJXMSY.2021.04.0041 |
| [14] |
IMSLAND F, FENG C G, BOIJE H, BED'HOM B, FILLON V, DORSHORST B, RUBIN C J, LIU R R, GAO Y, GU X R, WANG Y Q, GOURICHON D, ZODY M C, ZECCHIN W, VIEAUD A, TIXIER-BOICHARD M, HU X X, HALLBOOK F, LI N, ANDERSSON L. The rose-comb mutation in chickens constitutes a structural rearrangement causing both altered comb morphology and defective sperm motility[J]. PLoS Genetics, 2012, 8(6): e1002775. DOI:10.1371/journal.pgen.1002775 |
| [15] |
WRIGHT D, BOIJE H, MEADOWS J, BED'HOM B, GOURICHON D, VIEAUD A, TIXIER-BOICHARD M, RUBIN C J, IMSLAND F, HALLBOOK F, ANDERSSON L. Copy number variation in intron 1 of SOX5 causes the pea-comb phenotype in chickens[J]. PLoS Genetics, 2009, 5(6): e1000512. DOI:10.1371/journal.pgen.1000512 |
| [16] |
MORO C, CORNETTE R, VIEAUD A, BRUNEAU N, GOURICHON D, BED'HOM B, TIXIER-BOICHARD M. Quantitative effect of a CNV on a morphological trait in chickens[J]. PLoS One, 2015, 10(3): e118706. DOI:10.1371/journal.pone.0118706 |
| [17] |
SOMES R G. Linkage relationships in domestic fowl[J]. The Journal of Heredity, 1973, 64(4): 217-221. DOI:10.1093/oxfordjournals.jhered.a108392 |
| [18] |
DORSHORST B, HARUN-OR-RASHID M, BAGHERPOOR A J, RUBIN C J, ASHWELL C, GOURICHON D, TIXIER-BOICHARD M, HALLBOOK F, ANDERSSON L. A genomic duplication is associated with ectopic eomesodermin expression in the embryonic chicken comb and two duplex-comb phenotypes[J]. PLoS Genetics, 2015, 11(3): e1004947. DOI:10.1371/journal.pgen.1004947 |
| [19] |
PEARSON T A. How to interpret a genomewide association study[J]. Jama-Journal of the American Medical Association, 2008, 299(11): 1335-1344. DOI:10.1001/jama.299.11.1335 |
| [20] |
魏华, 李小兵. 不同冠型公鸡精液品质的对比试验报告[J]. 当代畜牧, 2010(9): 34-36. WEI H, LI X B. Comparative test report on semen quality of cocks with different crested types[J]. Contemporary Animal Husbandry, 2010(9): 34-36. |
| [21] |
CRAWFORD R D, SMYTH J, ROBERT J R. Semen quality and the gene for rose comb in the domestic fowl[J]. Poultry Science, 1964, 43(6): 1551-1557. DOI:10.3382/ps.0431551 |
| [22] |
ZHANG Y, ZHANG H, ZHANG B, LING Y, ZHANG H. Identification of key HIF-1alpha target genes that regulate adaptation to hypoxic conditions in Tibetan chicken embryos[J]. Gene, 2020, 729: 144321. DOI:10.1016/j.gene.2019.144321 |
| [23] |
WU P, YAN J, LAI Y C, NG C S, LI A, JIANG X Y, ELSEY R M, WIDELITZ R, BAJPAI R, LI W H, CHUONG C M. Multiple regulatory modules are required for scale-to-feather conversion[J]. Molecular Biology and Evolution, 2018, 35(2): 417-430. DOI:10.1093/molbev/msx295 |
| [24] |
FOOT N J, GEMBUS K M, MACKENZIE K, KUMAR S. Ndfip2 is a potential regulator of the iron transporter DMT1 in the liver[J]. Scientifi c Reports, 2016, 6: 24045. DOI:10.1038/srep24045 |
| [25] |
NG C C, YEW P Y, PUAH S M, KRISHNAN G, YAP L F, TEO S H, LIM P H, GOVINDARAJU S, RATNAVELU K, SAM C K, TAKAHASHI A, KUBO M, KAMATANI N, NAKAMURA Y, MUSHIRODA T. A genome-wide association study identifies ITGA9 conferring risk of nasopharyngeal carcinoma[J]. Journal of Human Genetics, 2009, 54(7): 392-397. DOI:10.1038/jhg.2009.49 |
| [26] |
LIU X, YANG P, HAN L, ZHOU Q, QU Q S, SHI S Y. The ncRNA-mediated overexpression of ferroptosis-related gene EMC2 correlates with poor prognosis and tumor immune infiltration in breast cancer[J]. Frontiers in Oncology, 2021, 11: 777037. DOI:10.3389/fonc.2021.777037 |
| [27] |
ZHAO Y, SONG K, ZHANG Y, XU H T, ZHANG X P, WANG L, FAN C F, JIANG G Y, WANG E H. TMEM17 promotes malignant progression of breast cancer via AKT/GSK3beta signaling[J]. Cancer Management and Research, 2018, 10: 2419-2428. DOI:10.2147/CMAR.S168723 |
| [28] |
LIU C X, YIN R X, SHI Z H, DENG G X, ZHENG P F, WEI B L, GUAN Y Z. EHBP1 SNPs, their haplotypes, and gene-environment interactive effects on serum lipid levels[J]. ACS Omega, 2020, 5(13): 7158-7169. DOI:10.1021/acsomega.9b03522 |
| [29] |
PATTURAJAN M, WEI X, BEREZNEY R, CORDEN J L. A nuclear matrix protein interacts with the phosphorylated C-terminal domain of RNA polymerase Ⅱ[J]. Molecular and Cellular Biology, 1998, 18(4): 2406-2415. DOI:10.1128/MCB.18.4.2406 |
| [30] |
JIANG B, ZHOU L, LU J, WANG Y Z, LIU C X, ZHOU W X, GUO J C. Elevated TIAM2 expression promotes tumor progression and is associated with unfavorable prognosis in pancreatic cancer[J]. Scandinavian Journal of Gastroenterology, 2021, 56(1): 59-67. DOI:10.1080/00365521.2020.1853806 |
| [31] |
SAUDOU F, HUMBERT S. The biology of Huntingtin[J]. Neuron, 2016, 89(5): 910-926. DOI:10.1016/j.neuron.2016.02.003 |
| [32] |
LUAN Z, ZHANG Y, LU T, RUAN Y, ZHANG H Y, YAN J, LI L Z, SUN W, WANG L F, YUE W H, ZHANG D. Positive association of the human STON2 gene with schizophrenia[J]. Neuroreport, 2011, 22(6): 288-293. DOI:10.1097/WNR.0b013e328345ac22 |
| [33] |
闫峰, 王效民, 袁思波, 马全明, 韩慧平. PRKCD和ASK1在人髓系白血病U937细胞分化过程中的作用[J]. 南方医科大学学报, 2015, 35(1): 17-22. DOI:10.3969/j.issn.1673-4254.2015.01.04 YAN F, WANG X M, YUAN S B, MA Q M, HAN H P. Role of PRKCD and ASK1 in U937 cell differentiation[J]. Journal of Southern Medical University, 2015, 35(1): 17-22. DOI:10.3969/j.issn.1673-4254.2015.01.04 |
| [34] |
BATRA J, HULTQUIST J F, LIU D, SHTANKO O, DOLLEN J V, SATKAMP L, JANG G M, LUTHRA P, SCHWARZ T M, SMALL G I, ARNETT E, ANANTPADMA M, REYES A, LEUNG D W, KAAKE R, HAAS P, SCHMIDT C B, SCHLESINGER L S, LACOUNT D J, DAVEY R A, AMARASINGHE G K, BASLER C F, KROGAN N J. Protein interaction mapping identifies RBBP6 as a negative regulator of ebola virus replication[J]. Cell, 2018, 175(7): 1917-1930. DOI:10.1016/j.cell.2018.08.044 |
| [35] |
LI L, CHEN Q, FENG C, JIN Y P, XIA S D. Aberrant expression of TNRC6a and miR-21 during myocardial infarction[J]. 3 Biotech, 2019, 9(7): 285. DOI:10.1007/s13205-019-1812-7 |
| [36] |
PLATZER K, STICHT H, EDWARDS S L, ALLEN W, ANGIONE K M, BONATI M T, BRASINGTON C, CHO M T, DEMMER L A, FALIK-ZACCAI T, GAMBLE C N, HELLENBROICH Y, IASCONE M, KOK F, MAHIDA S, MANDEL H, MARQUARDT T, MCWALTER K, PANIS B, PEPLER A, PINZ H, RAMOS L, SHINDE D N, SMITH-HICKS C, STEGMANN A P, STOBE P, STUMPEL C, WILSON C, LEMKE J R, DONATO N D, MILLER K G, AMRA R. De Novo Variants in MAPK8IP3 cause intellectual disability with variable brain anomalies[J]. American Journal of Human Genetics, 2019, 104(2): 203-212. DOI:10.1016/j.ajhg.2018.12.008 |
(责任编辑 崔建勋)
 2022, Vol. 49
2022, Vol. 49