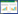文章信息
基金项目
- 国家重点研发计划项目(2021YFD300404);广东省国际科技合作项目(2023A0505050104);国家现代农业产业技术体系项目(CARS-41);广东省重点领域研发计划项目(2020B0202090004)
作者简介
- 王君雁(1999—),女,在读硕士生,研究方向为黄羽肉鸡营养调控,E-mail:1034449749@qq.com.
通讯作者
- 蒋守群(1971—),女,博士,研究员,研究方向为家禽营养与饲料科学,E-mail:jiangshouqun@gdaas.cn.
文章历史
- 收稿日期:2023-11-26
2. 广东省农业科学院动物科学研究所/猪禽种业全国重点实验室/农业农村部华南动物营养与饲料重点实验室/广东省畜禽育种与营养研究重点实验室,广东 广州 510640
2. Institute of Animal Science, Guangdong Academy of Agricultural Sciences/National Key Laboratory of Pig and Poultry Seed Industry/South China Key Laboratory of Animal Nutrition and Feed, Ministry of Agriculture and Rural Affairs/Guangdong Key Laboratory of Livestock and Poultry Breeding and Nutrition, Guangzhou 510640, China
姜黄素是一种从姜科植物姜黄和天南星科植物根茎中提取出来的多酚类疏水性化合物,具有抗氧化、抗炎、神经保护、免疫调节和代谢调控等多种生物学功能[1-5]。姜黄素在1815年被Vogel和Pelletier首次分离,并作为抗生素的替代品应用于临床治疗[6],其可通过消化道、呼吸道和血液进入体内,但主要在肠道内进行吸收。研究表明姜黄素主要通过调节肠道微生物菌群结构和免疫形态来发挥其生物学作用[7],游君怡等[8]研究表明,肠道微生物可通过脑- 肠轴调节机制影响生殖激素、血清代谢物和炎症反应等相关代谢途径,推测姜黄素能通过肠道微生物影响家禽的繁殖性能。姜黄素的代谢方式包括Ⅰ相还原代谢、Ⅱ相还原代谢、催化氧化和自身氧化,其最主要的代谢方式是Ⅰ相还原代谢和Ⅱ相还原代谢,并且各组织、细胞以及血液中最终产物多数是以Ⅰ相还原代谢产物发生葡萄糖醛酸化后存在[9]。姜黄素的代谢产物包括二氢姜黄素、四氢姜黄素、六氢姜黄素和八氢姜黄素,其中四氢姜黄素为体内主要代谢成分。生产上姜黄素因稳定性差、水溶性差,生物利用率低等问题被限制发展。因此为了提高姜黄素的生物利用度研发了多种生物制剂,包括常规的膜制剂、纤维制剂、乳制剂、水凝胶制剂以及新型的纳米制剂等将姜黄素定向送入靶器官,发挥其最大的生物效应[10]。
家禽的繁殖性能受多种因素调控,例如温度、湿度、添加剂、遗传、内分泌激素等,其中内分泌激素对下丘脑- 性腺轴的生物学作用直接影响家禽繁殖器官发育。研究表明,姜黄素可对下丘脑外周神经系统产生神经保护,保护下丘脑正常生理功能,从而提高动物的繁殖功能。同时姜黄素通过增加蛋清中免疫因子的含量来增强家禽子代的抗病力和孵化率[11-14]。提高动物的繁殖性有益于净化种群,因此姜黄素在家禽生产中具有广阔的应用前景。本文结合近年来国内外相关研究进展,从姜黄素生物活性、代谢途径、姜黄素制剂的研发以及在家禽繁殖调控中的作用等方面进行综述,以期为姜黄素在畜禽生产中的科学应用提供参考。
1 姜黄素的结构和代谢 1.1 姜黄素的化学结构姜黄素是一种橙黄色结晶粉末,属酸性多酚类化合物,不溶于水,易溶于乙醇、丙酮、碱液等有机溶剂[15-18]。姜黄素相对分子量较低,通过与特异性受体结合穿过细胞膜进入组织细胞[19]。姜黄素主要活性基团为对甲氧基[20],主要衍生物为去甲氧基姜黄素和双去甲氧基姜黄素[21],这2种衍生物次生代谢为二氢姜黄素、四氢姜黄素、六氢姜黄素和八氢姜黄素在体内发挥作用。
1.2 姜黄素的代谢途径姜黄素不易溶于水,在体内吸收利用率低,因此其在体内的吸收代谢途径一直以来被广泛研究。王浩楠等[22]通过总结姜黄素进入小鼠和大鼠体内后在不同部位的沉积量发现,大量的姜黄素在进入体内后都随粪便排出体外,少量随尿液排出。留在体内的姜黄素大部分在上消化道进行吸收代谢,少量进入血液、肝脏和小肠,且姜黄素的沉积量为小肠>肝脏>血液[9, 22-31],其具体在体内的吸收率见表 1[22]。姜黄素进入胃后和胃黏膜主细胞分泌的胃蛋白酶形成复合物,在缓解胃蛋白酶诱导的线粒体损伤[32-33]的同时刺激小肠分泌胃内因子,增加小肠吸收率。一部分姜黄素在小肠中形成还原产物与微生物结合,调节微生物菌群结构,并通过微生物- 肠- 脑轴实现肠道免疫和神经免疫的双向作用;另一部分通过肠系膜血液循环以及肠道微生物产生的雌激素和肠上皮细胞结合,增强肠黏膜免疫和抗氧化功能;姜黄素通过肝门静脉进入肝脏后发生首过效应,大部分被肝脏直接代谢排出,少部分与胆汁结合形成胆汁酸进入小肠,被小肠吸收的部分能通过肠系膜进入体循环,未吸收部分被排出体外。
1.3 姜黄素的代谢产物
姜黄素在中性或碱性溶液条件下易发生降解,当pH为弱碱性时容易发生自氧化[34-35]。姜黄素的代谢途径包括Ⅰ相还原代谢、Ⅱ相还原代谢、自身氧化和细胞内催化氧化。Ⅰ相还原代谢成为二氢姜黄素、四氢姜黄素、六氢姜黄素、八氢姜黄素和少量阿魏酸;Ⅰ相还原代谢产物与葡萄糖发生醛酸化或硫酸化形成Ⅱ相还原代谢[9],加快姜黄素在组织中的代谢[36]。姜黄素在消化道中经初步消化进入上消化道时会发生自身氧化,通过上皮细胞后发生Ⅰ相还原代谢、其代谢产物跟Ⅱ期代谢产物偶联进入下消化道,未被肠道吸收的姜黄素会通过大肠排出体外;被肠道吸收的姜黄素一部分与肠内微生物结合,进入肝脏与肝细胞结合被胆汁排泄;另一部分通过与血液中的白蛋白结合进入体循环[34, 37]。姜黄素在血浆中的主要代谢产物为四氢姜黄素。肝脏是第一个接触肠道组织的器官,肝脏中70% 的血液来自肠道。研究表明,姜黄素的代谢产物四氢姜黄素在肝脏、肾脏和大脑中的含量接近,且姜黄素可以通过血脑屏障,但其代谢物四氢姜黄素不能通过血脑屏障[38-39]。
1.4 姜黄素生物利用度优化为解决姜黄素生物利用度低的问题,临床上将姜黄素配制成多种制剂或配型,包括膜制剂、纤维制剂、乳制剂、水凝胶制剂等[9]。近年研究发现,纳米制剂可更准确地将姜黄素送至体内,提高其生物利用率。新型纳米制剂包括聚合物纳米颗粒、水凝胶、纳米乳液、纳米复合材料、纳米纤维、脂质体、纳米结构脂质载体、聚合物胶束、量子点、聚合物共混膜和基于纳米材料的姜黄素与其他抗菌剂的组合[40]。此外,姜黄素分别与胡椒碱、半萜类化合物、环糊精、葫芦巴膳食纤维、卵磷脂具有协同作用,可增加肠道对姜黄素的吸收率。新型纳米制剂更好地改善了姜黄素水溶性差的问题,能更准确地将姜黄素送到相应的组织中[41]。黄晓霞等[42]研究发现,负载姜黄素的玉米醇溶蛋白- 果胶纳米颗粒抗氧化活力比姜黄素乙醇溶液高2倍。虽然姜黄素的不同提取工艺会影响姜黄素的产率,但影响姜黄素在体内的吸收利用率主要与复配、剂型有关。优化后的姜黄素制剂能直接将姜黄素送到靶标部位,避免姜黄素进入肝门静脉后大部分被灭活。
2 姜黄素在家禽繁殖调控上的研究研究姜黄素对种鸡繁殖性能的影响及调控,对提高种鸡利用率和经济效益具有重要意义。大量研究表明,繁殖性能的降低主要是由氧化应激造成的激素紊乱、线粒体功能障碍和卵巢细胞衰老导致。其次,氧化应激带来的肠道损伤会影响肠内容物中的微生物平衡,并通过微生物- 肠脑轴影响垂体激素分泌,性类固醇在肠道中代谢后,通过肝肠循环进入卵巢组织中,且性类固醇与肠道微生物会产生互作作用而影响家禽繁殖性能。姜黄素作为良好的抗氧化和神经保护剂,能够缓解氧化应激带来的肠道紊乱和内分泌失调,进而提高蛋鸡和种鸡的产蛋能力、孵化性能和种公鸡的精子活力。目前关于姜黄素在家禽下丘脑- 垂体- 性腺轴负反馈调节机制和颗粒细胞线粒体功能障碍研究相对较少,大部分集中在哺乳动物上。
2.1 姜黄素对家禽繁殖性能的调控机制下丘脑- 垂体- 性腺轴负反馈调节机制是繁殖内分泌调控的重要特点。下丘脑释放促性腺激素释放激素(GnRH)刺激垂体释放卵泡刺激素(FSH)和黄体生成素(LH),再作用于卵巢或睾丸组织,使其分泌孕酮(P4)、雌二醇(E2)或睾酮(TTE)。研究表明,肠道微生物可以直接刺激肠神经系统的传入神经元,通过迷走神经传入下丘脑中多巴胺能神经信号,从而刺激GnRH释放[43]。姜黄素主要是通过调节肠道微生物来发挥其生理作用,因此推测姜黄素也可通过脑- 肠轴直接影响下丘脑- 垂体- 性腺轴的调控机制。
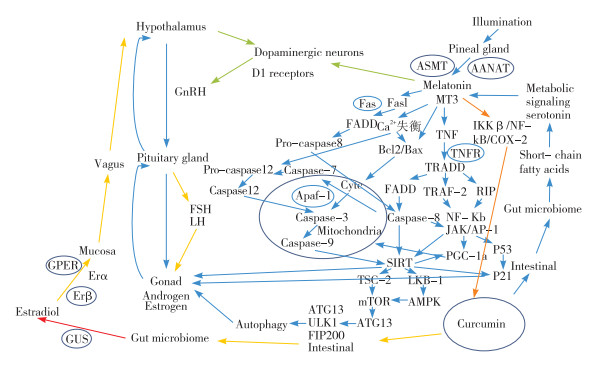
家禽的繁殖性能除了受下丘脑- 性腺轴的负反馈机制的调节外,也受到外部条件的影响。光照是影响繁殖性能最重要的因素之一。光照会影响松果腺分泌褪黑素,褪黑素具有很强的抗氧化性,能够减少活性氧(ROS)的生成,保护多巴胺能神经元的正常生理活性[44-47]。研究表明,家禽中褪黑素的受体类型为MT3,受肠道微生物的调控,MT3的前体物质存在于肠道中的血清素内;肠道微生物产生的短链脂肪酸会产生代谢信号转导血清素合成褪黑素,再通过线粒体途径、内质网途径以及生长因子代谢途径直接作用于卵巢和睾丸影响其凋亡和衰老。褪黑素可以降低ROS水平并缓解Ca2+失衡。褪黑素会通过增加抗凋亡基因Bax的表达来通过线粒体内膜释放的细胞色素C(CytC)缓解半胱天冬酶3和半胱天冬酶9逐级水解造成的卵巢凋亡;其次,褪黑素可与TNF受体结合,形成的TRADD再通过3条不同的通路调节细胞凋亡;最后,褪黑素通过和Fasl受体结合,激活半胱天冬酶8前体物质,形成半胱天冬酶7进入到内质网,与半胱天冬酶12的前体物质共同形成半胱天冬酶12调控半胱天冬酶3。3条通路最后形成的半胱天冬酶3和半胱天冬酶8共同调节SIRT/AMPK信号通路,降低巨噬细胞的吞噬功能,延缓卵巢和睾丸的衰老和凋亡[48-51]。此外,褪黑素与TNF受体结合,通过TRAF-2/NF-kB和RIP/JAK/AP-1通路影响P53和PGC-1α,影响线粒体发育及性腺组织的凋亡和衰老[52-53]。由于褪黑素可以通过IKKβ/NF-κB/COX-2信号通路增强姜黄素的抗凋亡作用[54],且姜黄素可以通过调节肠道微生物产生的短链脂肪酸促进褪黑素受体的表达。因此推测姜黄素和褪黑素可能通过互作影响下丘脑中的多巴胺受体分泌,促进性腺激素分泌。具体调控机制如图 1所示。
|
| 图 1 姜黄素对家禽繁殖性能的调控机制 Fig. 1 Regulatory mechanism of curcumin on reproductive performance in poultry |
2.2 姜黄素通过下丘脑- 性腺轴对家禽繁殖性能的调控作用
产蛋性能、蛋品质和子代孵化性能是评价家禽繁殖性能的重要指标,并受生殖激素的影响。研究表明,下丘脑- 垂体- 性腺轴在外周神经的刺激下调控卵巢和卵泡的生长发育[55-56],并影响卵泡生成素释放[57-60]。卵巢星状细胞是腺垂体中的非内分泌细胞,会影响卵泡细胞的发育,Schaaf等[61]选择卵泡星状(FS)TtT/GF小鼠垂体细胞进行试验,结果表明,姜黄素对TtT/GF细胞坏死的影响较小,但对凋亡影响较大,并且姜黄素通过影响FS细胞的发育间接影响垂体前叶的功能。Nishimura等[62]利用S100蛋白标记物标记腺垂体中FS细胞,并通过免疫组织化的方法确定碱性角蛋白在FS细胞中的定位。结果表明,腺垂体中的碱性细胞角蛋白是主要的FS细胞,并且角蛋白产生的层粘连蛋白是维持卵巢中相邻内分泌细胞和细胞索结构的基础。研究证明植物提取物可缓解肉鸡肝星状细胞活性降低和纤维化情况[63],但姜黄素对FS细胞的研究相对较少。
此外,下丘脑弓状核中多巴胺神经元的功能亚群也是影响繁殖性能的重要因素。在动物体内γ氨基丁酸(GABA)仅在下丘脑和垂体中间进行神经递质信号传递。研究表明,30%~40% 的中枢神经元以GABA为抑制性神经递质参与抗衰老等作用,且下丘脑的多巴胺神经元会在GABA的调控下刺激垂体分泌促乳素[64]。Cui等[65]用鱼藤酮建立大鼠帕金森病试验模型,并用姜黄素缓解,结果表明,姜黄素可通过Akt/Nrf2信号通路缓解帕金森病,并且改善鱼藤酮诱导的大鼠黑质致密部中的多巴胺能神经元氧化损伤。LYU等[66]使用RACE PCR在鸡脑中克隆2个D2样受体(cDRD2、cDRD4),使用Western Blot、免疫荧光等办法检测D2样受体在垂体中的表达。结果证明,cDRD4与小鼠DRD4的同源性为57%,且垂体中cDRD2/cDRD4的表达能诱导垂体细胞催乳素的表达。
FS细胞和下丘脑弓状核中多巴胺神经元均会影响卵泡发育,目前大部分姜黄素相关的研究集中在哺乳动物中,由于鸡下丘脑中的多巴胺受体与小鼠具有同源性,因此推测姜黄素也可能通过Akt/Nrf2信号通路来增加大脑中多巴胺神经元的分泌,维持下丘脑- 垂体- 性腺轴的正常功能。
2.3 姜黄素对卵巢抗氧化功能的调节作用氧化应激是卵巢凋亡与衰老的重要诱导因素。卵泡中包括生殖细胞(卵母细胞)、间质细胞及颗粒细胞,其中颗粒细胞中含有丰富的线粒体,是颗粒细胞和卵母细胞发育能量的主要来源,但线粒体功能障碍会出现卵泡细胞的凋亡和衰老,导致卵巢功能衰退。线粒体脂质过氧化会造成细胞色素C释放并进入线粒体细胞质,从而诱发半胱天冬酶3和半胱天冬酶9逐级水解造成细胞凋亡[67]。姜黄素是良好的抗氧化剂,可以缓解卵巢的氧化应激。Ye等[68]研究发现,姜黄素可缓解H2O2带来的繁殖性能下降。研究表明,线粒体功能障碍会导致性类固醇含量降低,受精率降低[69-70]。Zhang等[71]研究了姜黄素对多囊卵巢综合征大鼠高雄激素诱导的内质网应激和卵巢颗粒细胞凋亡作用,结果表明,姜黄素可缓解二氢睾酮诱导的内质网应激,通过α-XBP1通路改善卵巢功能。吴江等[72]发现,姜黄素可以通过Nod样受体信号通路、ErbB和FoxO信号通路缓解热应激带来的繁殖性能减退。
核因子E2相关因子1(Nrf1)和Nrf2是重要的抗氧化通路。研究表明,Nrf1主要是在正常生理状态下实现抗氧化功能,Nrf2主要是在应激状态下实现抗氧化功能,二者相互作用维持细胞的正常活性[73]。Sun等[74]建立了多囊卵巢综合征的体外模型,发现生物钟核心调节剂核受体REV-ERBα和REV-ERBβ及其激活剂SR9009的过表达促进了线粒体生物合成基因PGC-1α、Nrf1和TFAM转录,并抑制人卵巢颗粒细胞中线粒体的自噬,同时抑制颗粒细胞的凋亡。Wu等[75]对种公鸡腹腔注射H2O2制造氧化应激模型,并用姜黄素去缓解,结果表明,姜黄素可通过Nrf2信号通路和抗凋亡作用缓解H2O2诱导的精子氧化损伤和繁殖衰退。证明姜黄素可通过Nrf1和Nrf2信号通路来调控家禽卵巢的抗氧化和线粒体的修复功能。
沉默信息调节因子(Sirtuins,SIRT)为烟酰胺腺嘌呤二核苷酸依赖性组蛋白去乙酰化酶,可与组蛋白和多种转录因子结合,参与氧化应激、细胞凋亡、炎症和衰老等多种生理过程。SIRT1是SIRT家族成员之一,研究发现SIRT1介导的一些信号通路在卵泡发育和延缓卵巢衰老方面发挥重要作用。cAMP反应元件结合蛋白(CREB)是富含亮氨酸拉链结构的超家族成员,可抑制细胞凋亡。CREB蛋白是垂体和下丘脑之间重要的传递因子,也是影响下丘脑- 垂体- 性腺轴激素分泌的重要因子,SIRT1和CREB都是Nrf2调控的重要代谢蛋白。Azami等[76]研究发现,姜黄素可提高小鼠卵巢排卵相关生长分化因子9、骨形态发生蛋白15和SIRT1、SIRT3基因表达,促进小鼠卵母细胞细胞成熟、受精和胚胎发育,延缓衰老。袁翠等[77]报道,姜黄素能够提高SIRT1基因在蛋鸡卵巢中的表达,延缓蛋鸡卵巢组织细胞衰老和凋亡。因此,姜黄素可通过SIRT1/Nrf1-2信号通路缓解卵泡的凋亡及衰老,但Nrf2/CREB信号通路对家禽繁殖性能的影响研究相对较少。
2.4 姜黄素通过肠道微生物对家禽繁殖性能的调控作用肠道微生物是生物体各器官有效工作的重要组成部分, 可以调节中枢神经系统发育、稳态以及组织器官发育。大量研究发现,肠道菌群可以直接或间接调节生殖激素水平,进而影响畜禽繁殖性能[78-80]。芳香烃受体(AHR)是一种进化保守的细胞内受体,也是重要的配体依赖性转录因子,AHR可识别微生物组产生的多种配体,依赖配体活化的AHR可调控肠道微生物菌群结构及短链脂肪酸的合成。研究表明,AHR和雌激素受体参与肠道菌群调节,而且肠道微生物同样可以通过影响下丘脑- 垂体- 肾上腺轴来影响大脑的内分泌神经系统[81-83]。肠道微生物会影响促性腺激素的释放,性类固醇在代谢过程中会与肠腔内的微生物结合,通过血液进入肝脏,产生肝肠循环,最后在胆汁中代谢,通过体循环进入各个组织器官[84]。Zhang等[85]在切除卵巢的小鼠中添加姜黄素,检测肠道微生物16S RNA以及生殖激素的含量。结果表明,卵巢切除后引起的雌激素下降导致肠道菌群紊乱,但姜黄素可以上调血清中雌二醇含量,并调节微生物菌群结构。Saez等[86]选择鸡原代生殖细胞,使用芳烃受体拮抗剂进行阻断,探究确定已建立的AHR拮抗剂是否能对体外培养的鸡原代生殖细胞的增殖产生影响。结果表明,阻断AHR受体会抑制鸡原代生殖细胞的分化。
芳烃受体可通过对过氧化物酶体增殖物(PPAR)激活受体的负调节来影响机体生理代谢。PPAR激活受体受SIRT1/Nrf2和Nrf2/CREB信号通路共同调节糖脂代谢。Liu等[87]利用小鼠腹腔注射的方法,建立了脂肪肝模型,用聚乙二醇修饰的姜黄素进行刺激,并检测脂代谢相关指标。结果表明,聚乙二醇修饰的姜黄素可通过激活CREB抑制肝脏PPAR-γ/CD36通路来逆转高脂饮食诱导的肝脂肪变性。目前在家禽上大多数是研究芳烃受体与肠道健康的相互作用,关于姜黄素是否会通过芳烃受体改善肠道微生物菌群结构和糖脂代谢来影响家禽繁殖代谢的研究相对较少。
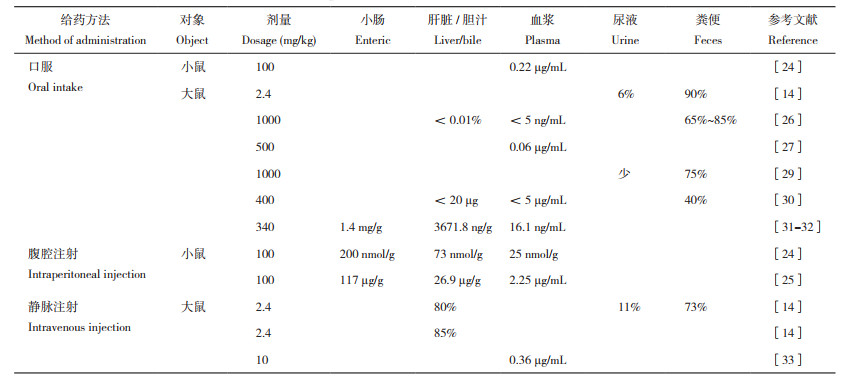
2.5 姜黄素对家禽繁殖性能的影响家禽的繁殖性能受到遗传、营养、饲养管理等因素影响。氧化应激会导致机体氧化损伤,降低家禽的繁殖性能[88]。Kazemizadeh等[89]研究发现,种公鸡精液浓度、总精子量、渐进运动和质膜完整性随饲粮姜黄素水平升高而线性改善。Liu等[13]研究发现,饲粮添加150 mg/kg的姜黄素可明显提高40周龄海兰蛋鸡产蛋性能和血清促卵泡激素、黄体生成素和雌二醇水平。夏卿等[90]研究发现,饲粮添加150 mg/kg的姜黄素可提高26周龄罗曼蛋鸡抗氧化功能、卵巢生殖激素受体mRNA相对表达量、血清生殖激素水平和小白卵泡数量,进而提高产蛋率,且最适添加量为150 mg/kg。高文[91]研究饲粮添加50~250 mg/kg姜黄素对高温环境下蛋鸡产蛋性能的影响,结果表明250 mg/kg姜黄素对蛋鸡产蛋率和卵泡发育效果最好。综上所述,添加一定水平姜黄素的饲粮可提高家禽的繁殖性能,这可能与姜黄素提高机体抗氧化能力、促进雌激素分泌有关。姜黄素对家禽繁殖性能的具体影响如表 2所示。
3 展望
姜黄素是一类重要的抗氧化剂,主要在肠道吸收和代谢并发挥生物活性。姜黄素在血液和不同组织种的存在形式不同,其主要的还原代谢产物为四氢姜黄素、六氢姜黄素和八氢姜黄素及少量阿魏酸。姜黄素在血液中的主要存在形式为四氢姜黄素,组织中的主要存在形式为六氢姜黄素。姜黄素的性质不稳定,易降解为次级产物,为提高其生物利用度,临床上将姜黄素配制成多种配型或制剂。新型的姜黄素纳米颗粒、胶束及姜黄素分别与胡椒碱、半萜类化合物、环糊精、葫芦巴膳食纤维、卵磷脂结合可增加肠道对姜黄素的吸收率,改善姜黄素的生物利用度。家禽的繁殖性能是净化种群的重要评价指标,繁殖调控是一个极其复杂的机制,HPG轴激素调控是最主要的繁殖调控方式,但外界环境同样能影响繁殖调控。前人研究表明,家禽繁殖性能减退主要是由氧化应激带来的性腺细胞衰老及凋亡造成,姜黄素作为抗氧化剂可缓解内质网、线粒体以及Ca2+失衡造成的氧化应激,并对卵巢星胶质细胞和多巴胺受体产生保护作用。多巴胺是下丘脑中的传递性神经,可影响GnRH分泌,LYU[66]的结果证明小鼠和鸡大脑中的多巴胺受体具有同源性,因此也推测姜黄素在鸡上具有与小鼠类似的作用。近年来关于多巴胺受体的研究多数在哺乳动物中,在家禽上的研究都相对较少,因此研究姜黄素通过保护下丘脑分泌多巴胺受体来影响家禽的繁殖性能有重大意义。临床上除多巴胺受体会对HPG轴产生影响外,肠道微生物也会通过脑- 肠道- 微生物影响HPG轴,因此肠道微生物也是机体重要的组成部分。姜黄素可与肠道微生物产生的芳烃结合调节,SIRT家族蛋白,因此推测姜黄素可能会在肠道内与芳烃受体结合,激活PPAR受体,负反馈调节Nrf2/SIRT1和Nrf2/CREB信号通路,保护多巴胺受体的正常分泌,最后通过HPG轴影响家禽的繁殖性能;也可能在肠道内与雌激素受体结合,通过血液进入卵巢,维持卵巢的正常发育。目前,姜黄素在家禽上的研究相对较少,主要在哺乳动物上研究。因此,本综述将为姜黄素在家禽繁殖方面的应用及相关调控机制提供理论依据。
| [1] |
SHAH D, SAVALIYA R, PATEL P, KANSARA K, PANDYA A, DHAWAN A, SINGH S. Curcumin Ag nanoconjugates for improved therapeutic effects in cancer[J]. International Journal of Nanomedicine, 2018, 13: 75-77. DOI:10.2147/IJN.S124696 |
| [2] |
RAI M, INGLE A P, PANDIT R, PARALIKAR P, ANASANE N, SANTOS C A D. Curcumin and curcuminloaded nanoparticles: Antipathogenic and antiparasitic activities[J]. Expert Review of Anti-Infective Therapy, 2020, 18(4): 367-379. DOI:10.1080/14787210.2020.1730815 |
| [3] |
PULIDO M M, MORNO F J, RAMIREZ T C, RAMIREZ T M. Curcumin and health[J]. Molecules, 2016, 21(3): 264. DOI:10.3390/molecules21030264 |
| [4] |
AGGARWAL B B, SUNDARAM C, MALANI N, ICHIKAWA H. Curcumin: The Indian solid gold[J]. Advances in Experimental Medicine and Biology, 2007, 595: 1-75. DOI:10.1007/978-0-387-46401-5-1 |
| [5] |
ALMEIDA M D, ROCHA B A D, FRANCISCO C R L, MIRANDA C G, SANTOS P D D F, ARAUJO P H H D, SAYER C, LEIMANN F V, GONCALVES O H, AMADO-BERSANI C A. Evaluation of the in vivo acute anti-inflammatory response of curcumin-loaded nanoparticles[J]. Food & Function, 2018, 9(1): 440-449. DOI:10.1039/c7fo01616f |
| [6] |
KUNNUMAKKARA A B, BORDOLOI D, HARSHA C, BANIK K, GUPTA S C, AGGARWAL B B. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways[J]. Clinical Science, 2017, 131(15): 1781-1799. DOI:10.1042/cs20160935 |
| [7] |
阮栋, 王一冰, 蒋守群, 郑春田. 姜黄素的生物活性及其调节动物肠道黏膜屏障功能的分子机制[J]. 动物营养学报, 2021, 33(4): 1801-1810. DOI:10.3969/j.issn.1006-267x.2021.04.001 RUAN D, WANG Y B, JIANG S Q, ZHENG C T. The biological activity of curcumin and its molecular mechanism that regulates intestinal mucosal barrier function in animals[J]. Chinese Journal of Animal Nutrition, 2021, 33(4): 1801-1810. DOI:10.3969/j.issn.1006-267x.2021.04.001 |
| [8] |
游君怡, 杜朝晖, 韩培源, 张宏兴, 王原, 梁国栋, 马云会, 史新娥, 胡建宏, 孙世铎, 李晓. 肠道微生物与母猪繁殖性能的相关性研究进展[J/OL]. 中国畜牧杂志, 1-13[2023-10-07]. DOI: 10.19556/j.0258-7033.20220803-02. YOU J Y, DU Z H, HAN P Y, ZHANG H X, WANG Y, LIANG G D, MA Y H, SHI X E, HU J H, SUN S D, LI X. Research progress on the correlation between gut microbiota and sow reproductive performance [J/OL]. Chinese Journal of Animal Science, 1-13[2023-10-07]. DOI: 10.19556/j.0258-7033.20220803-02. |
| [9] |
张琨, 郭振双, 王纯洁, 敖日格乐. 姜黄素和枯草芽孢杆菌混合型饲料添加剂对奶牛生产性能的调理作用[J]. 饲料研究, 2021, 44(6): 1-5. DOI:10.13557/j.cnki.issn1002-2813.2021.06.001 ZHANG K, GUO Z S, WANG C J, AO R G L. Conditioning effect of curcumin and Bacillus subtilis mixed feed additive on dairy cow production performance[J]. Feed Research, 2021, 44(6): 1-5. DOI:10.13557/j.cnki.issn1002-2813.2021.06.001 |
| [10] |
DONG X W, DENG L F, YAO S Q, WU W D, CAO J, SUN L, BAI Y C, LI H B, WENG X G, REN H C, REN W J. Protective effects of curcumin against thyroid hormone imbalance after gas explosioninduced traumatic brain injury via activation of the hypothalamic-pituitary-thyroid axis in male rats[J]. Environmental Science and Pollution Research International, 2022, 29(49): 74619-74631. DOI:10.1007/s11356-022-20943-2 |
| [11] |
MOHANTY C, SAHOO S K. Curcumin and its topical formulations for wound healing applications[J]. Drug Discovery Today, 2017, 22(10): 1582-1592. DOI:10.1016/j.drudis.2017.07.001 |
| [12] |
GAN R, LIU H Y, WU S F, HUANG R M, TANG Z X, ZHANG N, HU L M. Curcumin alleviates arsenic trioxide–induced inflammationand pyroptosis via the NF-κB/NLRP3 signaling pathway in the hypothalamus of ducks[J]. Biological Trace Element Research, 2022, 201(5): 2503-2511. DOI:10.1007/s12011-022-03321-4 |
| [13] |
LIU M J, LU Y L, GAO P, XIE X L, LI D F, YU D B, YU M L. Effect of curcumin on laying performance, egg quality, endocrine hormones, and immune activity in heat-stressed hens[J]. Poultry Science, 2020, 99(4): 72-78. DOI:10.1016/j.psj.2019.12.001 |
| [14] |
HOLDER G M, PLUMMER J L, RYAN A J. The metabolism and excretion of curcumin (1, 7-bis-(4-hydroxy-3-methoxyphenyl)-1, 6-heptadiene-3, 5-dione)in the rat[J]. Xenobiotica: The Fate of Foreign Compounds in Biological Systems, 1978, 8(12): 761-768. DOI:10.3109/00498257809069589 |
| [15] |
SAHNE F, MOHAMMADI M, NAJAFPOUR G D, MOGHADAMNIA A A. Enzyme-assisted ionic liquid extraction of bioactive compound from turmeric (Curcuma longa L.): Isolation, purification and analysis of curcumin[J]. Industrial Crops and Products, 2017,, 95: 686-694. DOI:10.1016/j.indcrop.2016.11.037 |
| [16] |
JIANG T, GHOSH R, CHARCOSSET C. Extraction, purification and applications of curcumin from plant materials-A comprehensive review[J]. Trends in Food Science & Technology, 2021, 112: 419-430. DOI:10.1016/j.tifs.2021.04.015 |
| [17] |
SOGI D S, SHARMA S, OBEROI D P S, WANI I A. Effect of extraction parameters on curcumin yield from turmeric[J]. Journal of Food Science and Technology, 2010, 47(3): 300-304. DOI:10.1007/s13197-010-0047-8 |
| [18] |
OTHMAN R, ABDURASID M A, MAHMAD N, FADZILLAH N A. Alkaline-based curcumin extraction from selected zingiberaceae for antimicrobial and antioxidant activities[J]. Pigment & Resin Technology, 2019, 48(4): 293-300. DOI:10.1108/prt-08-2018-0071 |
| [19] |
TODEN S, GOEL A. The Holy Grail of curcumin and its efficacy in various diseases: Is bioavailability truly a big concern?[J]. Restor Medical Spa, 2017, 6: 27-36. DOI:10.14200/jrm.2017.6.0101 |
| [20] |
SINGH A, SINGH J V, RANA A, BHAGAT K, GULATI H K, KUMAR R, SALWAN R, BHAGAT K, KAUR G, SINGH N, KUMAR R, SINGH H, SHARMA S, BEDI P M S. Monocarbonyl curcumin-based molecular hybrids as potent antibacterial agents[J]. ACS Omega, 2019, 4(7): 11673-11684. DOI:10.1021/acsomega.9b01109 |
| [21] |
TSUDA T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives[J]. Food & Function, 2018, 9(2): 705-714. DOI:10.1039/c7fo01242j |
| [22] |
王浩楠, 邱哲瀚, 田文妮, 肖杰. 姜黄素体内吸收代谢及与肠道菌群互作研究进展[J]. 中国食品学报, 2023, 23(2): 353-364. DOI:10.16429/j.1009-7848.2023.02.034 WANG H N, QIU Z H, TIAN W N, XIAO J. Research progress on curcumin's absorption, metabolism and interaction with intestinal flora[J]. Journal of Chinese Institute of Food Science and Technology, 2023, 23(2): 353-364. DOI:10.16429/j.1009-7848.2023.02.034 |
| [23] |
ALARCON P, GONZALEZ M, CASTRO E. The role of gut microbiota in the regulation of the immune response[J]. Revista Medical De Chile, 2016, 144(7): 910. DOI:10.4067/s0034-98872016000700013 |
| [24] |
PERKINS S, VER SCHOYLE R D, HILL K, PARVEEN I, THREADGILL M D, SHARMA R A, WILLIAMS M L, STEWARD W P, GESCHER A J. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+mouse, a model of familial adenomatous polyposis[J]. Cancer Epidemiol Biomarkers Prevention, 2002, 11(6): 535-540. |
| [25] |
PAN M H, HUANG T M, LIN J K. Biotransformation of curcumin through reduction and glucuronidation in mice[J]. Drug Metabolism & Disposition, 1999, 27(4): 486-494. DOI:10101144.
|
| [26] |
WAHLSTRÖM B, BLENNOW G. A study on the fate of curcumin in the rat[J]. Acta Pharmacol Toxicol, 1978, 43(2): 86-92. DOI:10.1111/j.1600-0773.1978.tb02240.x |
| [27] |
YANG K Y, LIN L C, TSENG T Y, WANG S C, TSAI T H. Oral bioavailability of curcumin in rat and the herbal analysis from curcuma longa by LC MS/MS[J]. Chromatogr B Analyt Technology Biomed Life Science, 2007, 853(1/2): 183-189. DOI:10.1016/j.jchromb.2007.03.010 |
| [28] |
AGGARWAL B B, SUNDARAM C, MOSLEY C A. The molecular targets and therapeutic uses of curcumin in health and disease[M]. Berlin: Springer Science & Business Media, 2007. DOI: 10.1007/978-0-387-46401-5.
|
| [29] |
SHARMA R A, STEWARD W P, GESCHER A J. Pharmacokinetics and pharmacodynamics of curcumin[J]. Advances in Experimental Medicine & Biology, 2007, 595(6): 453. DOI:10.1007/978-0-387-46401-5-20 |
| [30] |
RAVINDRANATH V, CHANDRASEKHARA N. Absorption and tissue distribution of curcumin in rats[J]. Toxicology, 1980, 16(3): 259-265. DOI:10.1016/0300-483x(80)90122-5 |
| [31] |
MARCZYLO T H, VERSCHOYLE R D, COOKE D N, MORAZZONI P, STEWARD W P, GESCHER A J. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine[J]. Cancer Chemotherapy & Pharmacology, 2007, 60(2): 171-177. DOI:10.1007/s00280-006-0355-x |
| [32] |
MARCZYLO T H, STEWARD W P, GESCHER A J. Rapid analysis of curcumin and curcumin metabolites in rat biomatrices using a novel ultraperformance liquid chromatography (UPLC) method[J]. Journal of Agricultural and Food Chemistry, 2009, 57(3): 797-803. DOI:10.1021/jf803038f |
| [33] |
SHOBA G, JOY D, JOSEPH T, MAJEED M, RAJENDRAN R, SRINIVAS P. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers[J]. Planta Medica, 1998, 64(4): 353-356. DOI:10.1055/s-2006-957450 |
| [34] |
HEGER M, GOLEN R F V, BROEKGAARDEN M, MICHEL M C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer[J]. Pharmacological Reviews, 2013, 68: 222-307. DOI:10.1124/pr.110.004044 |
| [35] |
PELIKH O, KECK C M. Hair follicle targeting and dermal drug delivery with curcumin drug nanocrystals—Essential influence of excipients[J]. Nanomaterials, 2020, 10(11): 2323. DOI:10.3390/nano10112323 |
| [36] |
WANG Y J, PAN M H, CHENG A L, LIN L L, HO Y S, HSIEH C Y, LIN J K. Stability of curcumin in buffer solutions and characterizationof its degradation products[J]. Journal of Pharmaceutical Biomed Analysis, 1997, 15(12): 1867-1876. DOI:10.1016/s0731-7085(96)02024-9 |
| [37] |
徐春明, 刘亚, 陈莹莹, 闫紫珊, 马慧鋆. 姜黄素生理活性、代谢以及生物利用度的研究进展[J]. 中国食品添加剂, 2016, 151(9): 203-210. XU C M, LIU Y, CHENG Y Y, YAN Z S, MA H Y. Research progress on physiological activity, metabolism and bioavailability of curcumin[J]. Chinese Food Additives, 2016, 151(9): 203-210. |
| [38] |
CAS D M, GHIDONI R. Dietary curcumin: Correlation between bioavailability and health potential[J]. Nutrients, 2019, 11: 2147. DOI:10.3390/nu11092147 |
| [39] |
WANG J J, YU X J, ZHANG L, WANG L, PENG Z H, CHEN Y. The pharmacokinetics and tissue distribution of curcumin and its metabolites in mice[J]. Biomedical Chromatography, 2018(42): 109-112. DOI:10.1002/bmc.4267 |
| [40] |
郭荧辉, 李洪军, 邢根安, 谢兆华, 韩薇, 张文振, 史佳龙, 贺稚非. 姜黄素的提取、生理特性及其在肉制品与鲜肉中的应用研究进展[J/OL]. 食品与发酵工业, 1-8[2023-09-07]. DOI: 10.13995/j.cnki.11-1802/ts.032906. GUO Y H, LI H J, XING G A, XIE Z H, HAN W, ZHANG W Z, SHI J L, HE Z F. Research progress on the extraction and physiological characteristics of curcumin and its application in meat products and fresh meat[J/OL]. Food and Fermentation Industries, 1-8[2023-09-07]. DOI: 10.13995/j.cnki.11-1802/ts.032906. |
| [41] |
WONG K E, NGAI S C, CHAN K G, LEE L H, GOH B H, CHUAH L H. Curcumin nanoformulations for colorectal cancer: A review[J]. Frontiers in Pharmacology, 2019, 10: 152. DOI:10.3389/fphar.2019.00152 |
| [42] |
黄晓霞, 黄旭琳, 胡坤. 负载姜黄素的玉米醇溶蛋白- 果胶纳米颗粒制备及抗氧化活性研究[J]. 广东农业科学, 2015, 42(18): 88-92, 2. DOI:10.16768/j.issn.1004-874X.2015.18.009 HUANG X X, HUANG X L, HU K. Preparation and antioxidant activity of zein/pectinnanoparticles loading curcumin[J]. Guangdong Agricultural Sciences, 2015, 42(18): 88-92, 2. DOI:10.16768/j.issn.1004-874X.2015.18.009 |
| [43] |
GALLAND L. The gut microbiome and the brain[J]. Journal of Medicinal Food, 2014, 17(12): 1261-1272. DOI:10.1126/science.abp9960 |
| [44] |
MARTINEZ DE L A ESCAL ER A G, GALLO F, CHOI A L, WEINER R I. Dopaminergic regulation of the GT1 gonadotropin-releasing hormone (GnRH) neuronal cell lines: Stimulation of GnRH release via D1-receptors positively coupled to adenylate cyclase[J]. Endocrinology, 1992, 131(6): 2965-2971. DOI:10.1210/endo.131.6.1280208 |
| [45] |
DAIRAGHI L, CONSTANTIN S, OH A, SHOSTAK D, WRAY S. The dopamine D4 receptor regulates gonadotropin-releasing hormone neuron excitability in male mice[J]. Neurological Sciences, 2022, 9(2): 421-432. DOI:10.1523/ENEURO.0461-21.2022 |
| [46] |
PARK S K, SEONG R K, KIM J A, SON S J, KIM Y H, YOKOZAWA T, SHIN O S. Oligonol promotes anti-aging pathways via modulation of SIRT1-AMPK-autophagy pathway[J]. Nutrition Research and Practice, 2016, 10(1): 3-10. DOI:10.4162/nrp.2016.10.1.3 |
| [47] |
LEEBOONNGAM T, PRAMONG R, SAE‐UNG K, GOVITRAPONG P. Neuroprotective effects of melatonin on amphetamine-induced dopaminergic fiber degeneration in the hippocampus of postnatal rats[J]. Journal of Pineal Research, 2018, 64(3): e12456. DOI:10.1111/jpi.12456 |
| [48] |
HENDERSON H L, TOWNSEND J, TORTONESE D J. Direct effects of prolactin and dopamine on the gonadotroph response to GnRH[J]. Journal of Endocrinology, 2008, 197(2): 343-350. DOI:10.1677/JOE-07-0536 |
| [49] |
LIU L L, CHEN H P, JIN J, TANG Z B, YIN P Q, ZHONG D, LI G Z. Melatonin ameliorates cerebral ischemia/reperfusion injury through SIRT3 activation[J]. Life Sciences, 2019, 239: 117036. DOI:10.1016/j.lfs.2019.117036 |
| [50] |
DONG Y Y, ZHAO J, ZHU Q Y, LIU H Y, WANG J, LU W F. Melatonin inhibits the apoptosis of rooster Leydig cells by suppressing oxidative stress via AKT-Nrf2 pathway activation[J]. Free Radical Biology and Medicine, 2020, 160: 1-12. DOI:10.1016/j.freeradbiomed.2020.06.024 |
| [51] |
黄清松, 李红枝, 陈爱葵. 姬松茸多糖诱导HepG2细胞凋亡的线粒体通路研究[J]. 广东农业科学, 2013, 40(15): 162-163, 175. DOI:10.16768/j.issn.1004-874X.2013.15.068 HUANG Q S, LI H Z, CHENG A K. Mitochondrial pathway study of agaricus polysaccharides-induced apoptosis in HepG2 cells[J]. Guangdong Agricultural Sciences, 2013, 40(15): 162-163, 175. DOI:10.16768/j.issn.1004-874X.2013.15.068 |
| [52] |
KANG M A, KIM M S, KIM J Y, SHIN Y J, SONG J Y, JEONG J H. A novel pyrido-thieno-pyrimidine derivative activates p53 through induction of phosphorylation and acetylation in colorectal cancer cells[J]. International Journal of Oncology, 2015, 46(1): 342-350. DOI:10.3892/ijo.2014.2720 |
| [53] |
TAY V S Y, DEVARAJ S, KOH T, KE GUO, CRASTA K C, ALI Y S. Increased double strand breaks in diabetic β-cells with a p21 response that limits apoptosis[J]. Scientific Reports, 2019, 9(1): 19341. DOI:10.1038/s41598-019-54554-8 |
| [54] |
SHRESTHA S, ZHU J B, WANG Q, DU X H, LIU F, JIANG J N, SONG J, XING J S, SUN D D, HOU Q J, PENG Y L, ZHAO J, SUN X Z, SONG X S. Melatonin potentiates the antitumor effect of curcumin by inhibiting IKKβ/NF-κB/COX-2 signaling pathway[J]. International Journal of Oncology, 2017, 1249-1260. DOI:10.3892/ijo.2017.4097 |
| [55] |
JIANG T, GHOSH R, CHARCOSSET C. Extraction, purification and applications of curcumin from plant materials-A comprehensive review[J]. Trends in Food Science & Technology, 2021, 112: 419-430. DOI:10.1016/j.tifs.2021.04.015 |
| [56] |
SHARIFI S, FATHI N, MEMAR M Y, KHATIBI S M H, KHALILOV R, NEGAHDARI R, VAHED S Z, DIZAJ S M. Anti-microbial activity of curcumin nanoformulations: New trends and future perspectives[J]. Pharmacological Reviews, 2020, 34(8): 926-1946. DOI:10.1002/ptr.6658 |
| [57] |
范炜. 家禽繁殖性能相关分子机制研究[J]. 今日畜牧兽医, 2017(7): 14. FAN W. Study on molecular mechanisms related to poultry reproductive performance[J]. Today Animal Husbandry and Veterinary medicine, 2017(7): 14. |
| [58] |
ASKEW J A, GEORGIOU G C, SHARP P J, LEA R W. Localization of progesterone receptor in brain and pituitary of the ring dove: Influence of breeding cycle and estrogen[J]. Hormones & Behavior, 1997, 32(2): 105-113. DOI:10.1006/hbeh.1997.1411 |
| [59] |
YOUSEFVAND S, HAMIDI F. The role of ventromedial hypothalamus receptors in the central regulation of food intake[J]. International Journal of Peptide Research and Therapeutics, 2021, 27(2): 1-14. DOI:10.1007/s10989-020-10120-9 |
| [60] |
BI Y L, YANG S Y, WANG H Y, CHANG G B, CHEN G H. Folliclestimulating hormone is expressed in ovarian follicles of chickens and promotes ovarian granulosa cell proliferation[J]. Journal of Agricultural Sciences, 2021, 20(10): 9. DOI:10.1016/S2095-3119(21)63606-7 |
| [61] |
SCHAAF C, SHAN B, ONOFRI C, STALLA G K, ARZT E, SCHILLING T, PERONE M J, RENNER U. Curcumin inhibits the growth, induces apoptosis and modulates the function of pituitary folliculostellate cells[J]. Neuroendocrinology, 2010, 91(2): 200-210. DOI:10.1159/000287236 |
| [62] |
NISHIMURA S, YAMASHITA M, KANEKO T, KAWABATA F, TABATA S. Cytokeratin-positive folliculo-stellate cells in chicken adenohypophysis[J]. Animal Science Journal, 2017, 88(11). DOI:10.1111/asj.12866 |
| [63] |
TSAI M T, CHEN C Y, PAN Y H, WANG S H, MERSMANN H J, DING S T. Alleviation of carbon-tetrachloride-induced liver injury and fibrosis by betaine supplementation in chickens[J]. Evidence-Based Complementray and Alternative Medicine, 2015, 2015: 725379. DOI:10.1155/2015/725379 |
| [64] |
BROWN R S E, KOKAY I C, PHILLIPPS H R, YIP S H, GUSTAFSON P, WYATT A, LARSEN C M, KNOWLES P, LADYMAN S R, TISSIER P L, GRATTAN D R. Conditional deletion of the prolactin receptor reveals functional subpopulations of dopamine neurons in the arcuate nucleus of the hypothalamus[J]. The Journal of Neuroscience, 2016, 36(35): 21-29. DOI:10.1523/JNEUROSCI.1471-16.2016 |
| [65] |
CUI Q L, LI X, ZHU H C. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway[J]. Molecular Medicine Reports, 2016, 1381-1388. DOI:10.3892/mmr.2015.4657 |
| [66] |
LYU C, MO C H, LIU H K, WU C, LI Z Y, LI J, WANG Y J. Dopamine D2-like receptors (DRD2 and DRD4) in chickens: Tissue distribution, functional analysis, and their involvement in dopamine inhibition of pituitary prolactin expression[J]. Gene, 2018, 651: 33-43. DOI:10.1016/j.gene.2018.01.087 |
| [67] |
PARADIES G, PETROSILLO G, PISTOLESE M, RUGGIERO F M. The effect of reactive oxygen species generated from the mitochondrial electron transport chain on the cytochrome C oxidase activity and on the cardiolipin content in bovine heart submitochondrial particles[J]. FEBS Letters, 2000, 466(2/3): 323-326. DOI:10.1016/s0014-5793(00)01082-6 |
| [68] |
YE N W, LYU Z P, DAI H J, HUANG Z W, SHI F X. Dietary alphalipoic acid supplementation improves spermatogenesis and semen quality via antioxidant and anti-Apoptotic effects in aged breeder roosters[J]. Theriogenology, 2021, 159: 20-27. DOI:10.1016/j.theriogenology.2020.10.017 |
| [69] |
URS D B S, WU W H, KOMRSKOVA K, POSTLEROVA P, LIN Y F, TZENG C R, KAO S H. Mitochondrial function in modulating human granulosa cell steroidogenesis and female fertility[J]. International Journal of Molecular Sciences, 2020, 21(10): 3592. DOI:10.3390/ijms21103592 |
| [70] |
KANSAKU K, ITAMI N, RYOUKA K M, SHIRASUNA K, KUWAYAMA T, IWATA H. Differential effects of mitochondrial inhibitors on porcine granulosa cells and oocytes[J]. Theriogenology, 2017, 103: 98. DOI:10.1016/j.theriogenology.2017.07.049 |
| [71] |
ZHANG Y L, WENG Y L, WANG D J, WANG R, WANG L H, ZHOU J J, SHEN S M, WANG H W, WANG Y. Curcumin in combination with aerobic exercise improves follicular dysfunction via inhibition of the hyperandrogen-induced IRE1α/XBP1 endoplasmic reticulum stress pathway in PCOS-like rats[J]. Oxidative Medicine and Cellular Longevity, 2021. DOI:10.1155/2021/7382900 |
| [72] |
吴江, 康恺, 李光辉, 唐淑艳, 赵一, 孙晨雨, 效梅, 安立龙. 姜黄素对热应激条件下蛋鸡卵巢组织circRNA表达谱的影响[J]. 中国兽医学报, 2020, 40(4): 833-841. DOI:10.16303/j.cnki.1005-4545.2020.04.29 WU J, KANG K, LI G H, TANG S Y, ZHAO Y, SUN C Y, XIAO M, AN L L. Effect of curcumin on circRNA expression profiling of layer hen ovary tissue under heat stress[J]. Chinese Journal of Veterinary Medicine, 2020, 40(4): 833-841. DOI:10.16303/j.cnki.1005-4545.2020.04.29 |
| [73] |
袁建新. 转录因子Nrf1/2在细胞应激中的作用机制及Nrf1D生化特性的鉴定[D]. 重庆: 重庆大学, 2021. DOI: 10.27670/d.cnki.gcqdu.2019.001000. YUAN J X. Mechanism of transcription factor Nrf1/2 in cellular stress and identification of biochemical properties of Nrf1[D]. Chongqing: Chongqing University, 2021. DOI: 10.27670/d.cnki.gcqdu.2019.001000. |
| [74] |
SUN L H, TIAN H, XUE S G, YE H J, XUE X, WANG R X, LIU Y, ZHANG C X, CHEN Q J, GAO S R. Circadian clock genes REVERBs inhibits granulosa cells apoptosis by regulating mitochondrial biogenesis and autophagy in polycystic ovary syndrome[J]. Frontiers in Cell and Developmental Biology, 2021(9): 658112. DOI:10.3389/fcell.2021(9):658112.658112 |
| [75] |
WU H Z, YE N W, HUANG Z W, LEI K, SHI F X, WEI Q W. Dietary curcumin supplementation relieves hydrogen peroxideinduced testicular injury by antioxidant and anti-apoptotic effects in roosters[J]. Theriogenology, 2023, 197: 46-56. DOI:10.1016/j.theriogenology.2022.10.038 |
| [76] |
AZAMI S H, NAZARIAN H, ABDOLLAHIFAR M A, EINI F, FARSANI M A, NOVIN M G. The antioxidant curcumin postpones ovarian aging in young and middle-aged mice[J]. Reproduction, Fertility and Development, 2020, 32(3): 292-303. DOI:10.1071/RD18472 |
| [77] |
袁翠, 苏柳玥, 吴倩玲, 罗鑫旭, 林玉, 吴江. 姜黄素对13月龄罗曼蛋鸡卵巢组织结构和Sirt1、Sirt2、Sirt3基因表达的影响[J]. 现代畜牧与兽医, 2022(9): 21-26. YUAN C, SU L Y, WU Q L, LUO X X, LIN Y, WU J. Effect of curcumin on ovarian tissue structure and Sirt1, Sirt2 and Sirt3 gene expression in 13-month-old Roman laying hens[J]. Modern Animal Husbandry and Veterinary Medicine, 2022(9): 21-26. |
| [78] |
NEUMAN H, DEBELIUS J W, KNIGHT R, KOREN O. Microbial endocrinology: The interplay between the microbiota and the endocrine system[J]. FEMS Microbiol Review, 2015, 39(4): 509-521. DOI:10.1093/femsre/fuu010 |
| [79] |
HE Y F, WANG Q Q, LI X, WANG G, ZHAO J X, ZHANG H, CHEN W. Lactic acid bacteria alleviate polycystic ovarian syndrome by regulating sex hormone related gut microbiota[J]. Food Function, 2020, 11(6): 5192-5204. DOI:10.1039/c9fo02554e |
| [80] |
杨曾乔, 丁雪梅, 百世平, 曾秋凤, 张克英, 王建萍. 不同产蛋水平肉种鸡繁殖性能、肠道组织形态、卵巢功能和盲肠微生物区系差异研究[J]. 动物营养学报, 2021, 33(1): 270-284. DOI:10.3969/j.issn.1006-267x.2021.01.028 YANG C Q, DING X M, BAI S P, ZENG Q F, ZHANG K Y, WANG J P. Differences in reproductive performance, intestinal tissue morphology, ovarian function and cecal microflora of broiler breeders at different egg laying levels[J]. Chinese Journal of Animal Nutrition, 2021, 33(1): 270-284. DOI:10.3969/j.issn.1006-267x.2021.01.028 |
| [81] |
LEE H U, PHERSON Z E M, TAN B, KORECKA A, PETTERSSON S. Host-microbiome interactions: The aryl hydrocarbon receptor and the central nervous system[J]. Journal of Molecular Medicine, 2017, 95(1): 29-39. DOI:10.1007/s00109-016-1486-0 |
| [82] |
CHEN L G, ZHANG W P, HUA J H, HU C Y, LAI N L S, QIAN P Y, LAM P K S, LAM J C W, ZHOU B S. Dysregulation of intestinal health by environmental pollutants: Involvement of the estrogen receptor and Aryl hydrocarbon receptor[J]. Environmental Science & Technology, 2018, 52(4): 2323-2330. DOI:10.1021/acs.est.7b06322 |
| [83] |
HUO R, ZENG B H, ZENG L, CHENG K, LI B, LUO Y Y, WANG H Y, ZHOU C J, FANG L, LI W X, NIU R, WEI H, XIE P. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis[J]. Frontiers in Cellular and Infection Microbiology, 2017, 7: 489. DOI:10.3389/fcimb.2017.00489 |
| [84] |
ORGANSKI A C, JORGENSEN J S, CROSS T W L. Involving the life inside: The complex endocrine regulation and the gut microbiota-ScienceDirect[J]. Current Opinion in Endocrine and Metabolic Research, 2021, 20: 100284. DOI:10.1016/j.coemr.2021.100284 |
| [85] |
ZHANG Z G, CHEN Y J, XIANG L H, WANG Z, XIAO G, HU J Q. Effect of curcumin on the diversity of gut microbiota in ovariectomized rats[J]. Nutrients, 2017, 9(10): 1146. DOI:10.3390/nu9101146 |
| [86] |
SAEZ J M P, BUSSMANN L E, BARANAO J L, BUSSMANN U A. Improvement of chicken primordial germ cell maintenance in vitro by blockade of the Aryl hydrocarbon receptor endogenous activity[J]. Cellular Reprogramming, 2016, 18(3): 154-161. DOI:10.1089/cell.2016.0015 |
| [87] |
LIU Y, CHENG F, LUO Y X, ZHAN Z, HU P, REN H, TANG H D, PENG M L. PEGylated curcumin derivative attenuates hepatic steatosis via CREB/PPAR-γ/CD36 pathway[J]. Biomed Research International, 2017, 2017(1): 1-11. DOI:10.1155/2017/8234507 |
| [88] |
张云波, 刘华忠, 谷毅鹏, 肖为, 罗萍. 乌贼墨多糖对环磷酰胺介导小鼠卵巢氧化应激损伤的干预效应[J]. 广东农业科学, 2015, 42(14): 90-93. DOI:10.16768/j.issn.1004-874X.2015.14.030 ZHANG Y B, LIU H Z, GU Y P, XIAO W, LUO P. Intervention effect of cuttlefish mopolysaccharide on cyclophosphamide-mediated ovarian oxidative stress injury in mice[J]. Guangdong Agricultural Sciences, 2015, 42(14): 90-93. DOI:10.16768/j.issn.1004-874X.2015.14.030 |
| [89] |
KAZEMIZADEH A, SHAHNEH A Z, ZEINOALDINI S, YOUSEFI S, YEGANEH M, ANSARI H, ZARBAKHT P, AMIR A. Effects of dietary curcumin supplementation on seminal quality indices and fertility rate in broiler breeder roosters[J]. British Poultry Science, 2019, 60(3): 256-264. DOI:10.1080/00071668.2019.1571165 |
| [90] |
夏卿, 高文, 陈玉霞, 谢婷婷, 张丽, 效梅, 安立龙. 姜黄素对罗曼蛋鸡产蛋性能、卵泡发育和抗氧化功能的影响[J]. 黑龙江畜牧兽医, 2023(17): 98-103. DOI:10.13881/j.cnki.hljxmsy.2023.01.0104 XIA Q, GAO W, CHENG Y X, XIE T T, ZHANG L, XIAO M, AN L L. Effect of curcumin on egg laying performance, follicular development and antioxidant function of Roman laying hens[J]. Heilongjiang Animal Science and Veterinary Medicine, 2023(17): 98-103. DOI:10.13881/j.cnki.hljxmsy.2023.01.0104 |
| [91] |
高文. 姜黄素对高温环境下罗曼蛋鸡产蛋率及卵泡发育的影响[D]. 湛江: 广东海洋大学, 2022. DOI: 10.27788/d.cnki.ggdhy.2020.000206. GAO W. Effect of curcumin on egg production rate and follicle development of Roman laying hens under high temperature environment[D]. Zhanjiang: Guangdong Ocean University, 2022. DOI: 10.27788/d.cnki.ggdhy.2020.000206. |
| [92] |
张帅, 付康, 杨蕾, 张宝月, 唐利军, 舒刚, 阳刚, 杨仕群. 姜黄素与百里香酚联合应用对丰岩乌骨鸡产蛋性能与抗氧化能力的影响[J]. 饲料工业, 2023, 44(23): 49-54. DOI:10.13302/j.cnki.fi.2023.23.008 ZHANG S, FU K, YANG L, ZHANG B Y, TANG L J, SHU G, YANG G, YANG S Q. Effects of the combined application of curcumin and thymol on the egg laying performance and antioxidant capacity of Fengyan black-bone chickens[J]. Feed Industry, 2023, 44(23): 49-54. DOI:10.13302/j.cnki.fi.2023.23.008 |
责任编辑 陈丽娥
 2024, Vol. 51
2024, Vol. 51