文章信息
基金项目
- 黑龙江大学大学生创新创业训练计划项目(2023004);黑龙江省高校基本科研业务费黑龙江大学专项资金项目(2022-KYYWF-1093,2021-KYYWF-0025)
作者简介
- 宋鸽(1986—),女,硕士,讲师,研究方向为土壤有机质生理活性,E-mail:1093972779@qq.com.
文章历史
- 收稿日期:2023-11-30
2. 黑龙江大学中俄学院,黑龙江 哈尔滨 150080
2. Sino-Russia Institute, Heilongjiang University, Harbin 150080, China
腐殖质(Humic substances,HS)是陆地生态系统的重要组成部分,是热力学上性质稳定的有机质保护形式,占土壤有机质(Soil organic matters,SOM)总量60%,腐殖化作用是地球上仅次于光合作用的第二大有机过程[1-2]。由于原始转化材料来源复杂、微生物群落、环境参数、转化时间差异和HS的多分散性等原因,至今尚未明确HS的分子结构。HS是小分子和其他异构聚合物通过疏水相互作用(即范德华力,π-π和CH-π)和氢键缔合在一起的天然高分子异构混合物,分子量为500~1 000 000 Da,包含不同的官能团,如酚基、羧基、醌基、烯醇基和醚基等[3-4]。近期研究表明,HS含有自然界常见生物分子所没有的特殊结构特征,其特点是C = O基团分布于高度取代芳族化合物和高度共轭体系的脂肪族化合物中,且含有C-O烷基链[5]。在溶液中,这些分子趋于形成稳定的分子自组装体,从而呈现新的化学性质[6],并影响其生物活性。
HS天然高分子异构混合物能够影响植物多种生理过程,包括养分吸收、蛋白质组、代谢组变化和差异基因表达等[7-13]。HS超分子结构进入细胞的过程和机制一直是学者关注的热点问题。Vaughan等[14]在采用14C同位素标记胡敏酸(Humic acid,HA)和富里酸(Fulvic acid,FA)的研究中发现,在HS和豌豆(Pisum sativum)根系作用3 h内HA和FA大量结合在细胞壁上,18 h后HA和FA转变成了植物细胞内可溶性组分,而且大量HA结合在细胞壁上,更多的FA进入细胞内。原因是HS同细胞壁组分通过氢键和疏水作用有效结合,吸附在细胞表面,而FA分子量相对较小,因此被转运进入细胞。Kulikova等[15]利用氚标记HS的研究中也证实HS组分在根质外体中累积。HS在细胞壁表面的沉积可降低根系的水力传导率(Lpr),阻碍根系的水分吸收,影响地上部的生长,降低蒸腾作用,此种现象即胶体应激(Colloidal stress)。胶体应激会引起短暂的轻度胁迫,可能触发与HS对植物保护作用相关的一系列生化和生理学过程,这种类型的轻度胁迫又称良性应激(Eustress)[16],可作为植物适应非生物胁迫的应答机制。Berbara等[17]利用显微镜观察到HA在根部团聚的现象,13C-NMR研究证实HA与根表面存在相互作用,而且在根表面形成团聚物的HA结构复杂性较低。此外,叶和根组织中ROS的产生也验证了HA和根细胞相互作用,即胶体应激触发了抗氧化代谢酶的活性。研究还认为HA和根细胞的相互作用与HS作用模式有关,可能通过调节氧化还原平衡(Redox homeostasis)和HS诱导的其他代谢过程来控制根和地上部的生长发育[18-19]。HS通过多条复杂的信号通路和串扰机制调节根和地上部的生长发育,包括IAA、JA、NO、ABA、Ca2+、ROS和CTK等,通过触发多条响应HS的信号通路传导、信号通路相互间的串扰及信号通路的整合来动态调节植物根和地上部的生理过程。本文参考诸多学者在相关领域的研究成果,总结和分析了HS促进植物根和地上部生长发育过程中主要的信号通路传导和串扰机制,以期为HS生理活性机制的研究及农业生产应用提供理论参考。
1 HS触发根生长发育的信号通路传导和串扰机制研究表明,HS的明显特征是促进根伸长、根毛形成和侧根发育[20-22](图 1),增加根系对水分和营养元素的吸收面积。HS具有IAA活性[23-25]。NO信号通路参与调控HS诱导的根细胞质膜H+-ATPase活性,导致其活性增加[26-27]。HS还能够影响根细胞中ROS代谢变化[28-29],而ROS通常被认为是有氧代谢产生的有毒化学物质[30]。在HS和根细胞互作过程中,HS触发多条信号通路及相互间串扰过程,在细胞和亚细胞水平上参与控制植物生长和发育等代谢过程[31-34]。

|
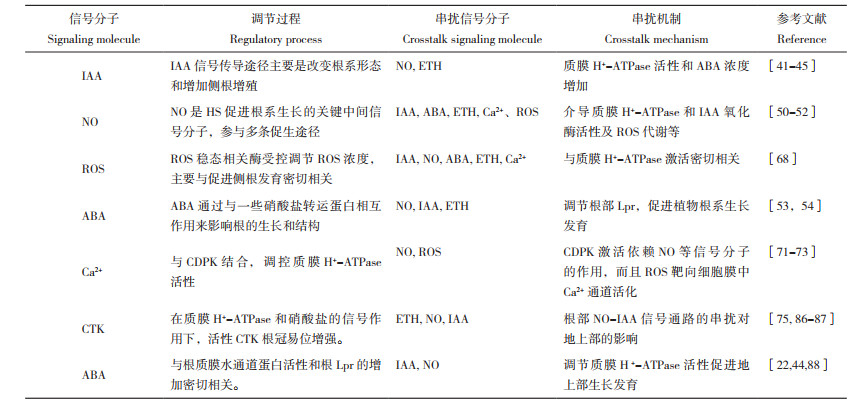
| 植物在 Hoagland 溶液中生长,最后 2 d 用 HS 处理;A:左侧为 HS 处理过的植物,右侧为未用 HS 处理的植物;B:未经处理的植物;C:HS 处理的植物 Plants were grown in Hoagland solution and treated with HS for the last 2 days; A: Plant treated with HS on the left, untreated plant on the right; B: Untreated plant; C: Plant treated with HS. rp: root primordia; rh: root hair 图 1 小麦幼苗根尖顶端0~20 mm区域表面SEM照片[22] Fig. 1 SEM micrographs of the 0-20 mm region behind the root tips of wheat seedlings surface[22] |
1.1 IAA信号通路及串扰信号分子
HS介导根系统发育过程涉及到的信号分子及其可能的串扰信号分子,如表 1所示。这些信号分子在HS介导的根系增厚、干重增加及次生根的发育过程中起重要作用[35]。研究认为,HA对根生长和结构的影响主要由IAA信号通路介导,HS包含的IAA组分可能会通过细胞受体启动细胞内信号传导[36-38]。IAA信号通路对根生长促进作用与质膜H+-ATPase活性密切相关,质膜H+-ATPase可能为IAA的主要作用靶点,其作用机制可用酸生长理论解释[39]。理论上,土壤环境中的植物根系分泌柠檬酸以分解HS的超分子结构,IAA作用于质膜H+-ATPase,将细胞内H+泵出细胞外,从而降低质外体pH,激活细胞壁松散酶,导致细胞壁松动,细胞体积膨胀。Zandonadi等[40]研究证实,HA处理植物时施用IAA抑制剂(PCIB)可避免HS介导的对根形态和侧根增殖的影响。Trevisan[41]利用IAA响应报告基因DR5 : : GUS证实,将HA应用于根部会导致质膜H+-ATPase活性和ABA浓度增加,IAA响应基因表达上调。然而近期研究表明,除IAA外的其他化合物可能通过引发内源性信号来控制HS介导的根系发育,此外,IAA响应基因(如IAA5和IAA19)对HS的应答具有选择性,因此NO和乙烯(Ethylene,ETH)信号通路也可能参与根生长发育的调节[42-45]。
|
1.2 信使NO通路及串扰信号分子
信使NO是参与植物根生长、侧根形成、细胞凋亡、防御代谢等的重要生物活性分子[46]。HS作用于幼苗侧根特定部位诱导NO的产生,且能通过调节根毛细胞中相关基因的表达来改变根系形态,从而增加根系的吸收面积[35, 47-48]。HS可能通过酸化胞内环境提高NO还原酶活性,从而诱导NO合成[49]。研究表明,堆肥和有机沉积物中提取的HS能够通过激活根细胞IAA和NO信号通路来诱导侧根增殖[50-52],同时引发根中IAA应答基因表达上调[50]。此外,根细胞中IAA和NO浓度的升高与HS介导根质膜H+-ATPase活性的增强密切相关[40, 50]。理论上HS被认为是外源IAA,通过靶向根质膜H+-ATPase和生成的NO交叉诱导调节根的生长和形态[16]。HS介导的根质膜H+- ATPase活性的增加与HS对植物生长促进作用的相关性一直都是研究的热点问题。Olaetxea等[47]研究发现,N, N’-二环己基碳二亚胺(N, N’-Dicyclohexylcarbodiimide, DCC)是质膜H+-ATPase的抑制剂,能够抑制沉积腐殖酸(Sedimentary humic acid, SHA)引起的根和地上部的生长,由此表明质膜H+-ATPase活化是SHA促进植物生长的关键。研究还证实,堆肥腐殖酸(Compost humic acids, CHA)介导的质膜H+-ATPase活化具有IAA和NO依赖性。NO清除剂(PTIO)抑制了IAA介导的质膜H+-ATPase活化,表明IAA的作用具有NO依赖性。然而,IAA抑制剂(PCIB)是否影响NO介导的质膜H+-ATPase活化未有研究报道。此外,PCIB和PTIO均不会影响主根长度,但会明显降低CHA介导的侧根出现和密度的增加[47]。Terrile等[51]的研究结果显示,在PCIB或PTIO存在下CHA处理的植物比对照植物具有更高的侧根发生率和密度,表明CHA对根发育的调控也可能涉及其他信号传导途径。Mora等[52]的研究表明,对根施加HA导致IAA浓度明显增加,这与根中NO和ETH浓度增加有关。尽管HA对根结构和侧根发育产生的影响可以通过其对IAA和NO信号通路的影响来解释,但HA介导的整个根干物质量增加与IAA、ETH和NO信号通路无关,而可能与HA促进根质膜H+-ATPase活性的增加密切相关。
NO-IAA信号的串扰方面,IAA可以促进不同植物在不同的试验条件下根细胞中NO的产生[52]。NO通过IAA受体TIR1区的特定部分(cys-180和cys-480)S- 亚硝化作用增强IAA的功能活性[50],还可以通过抑制IAA氧化酶活性来促进IAA的积累[51]。Mora等[52]研究表明在NO-IAA信号串扰参与HS对根发育和功能作用的情况下,SHA可促进根NO产生,通过IAA氧化酶抑制IAA降解,增加IAA的浓度。然而,该机制还与SHA对根IAA的优先作用(Prior effect)有关,即IAA根分布或IAA受体敏感性与随后对IAA功能(TIR1 / S- 亚硝基化)作用相容。此外,与NO信号无关的途径也参与SHA介导的根系IAA增加。
1.3 ABA依赖性信号通路及串扰信号分子SHA促进根生长的能力也可能与ABA依赖性信号通路有关,HS处理的植物根系中IAA、NO、ETH和ABA含量增加。研究证实,ABA和IAA含量增加在施用HS的黄瓜根系生长中起到重要作用,IAA抑制剂可抑制黄瓜次生根的发育,但HS保持了诱导其总根生物量增加的能力[52]。然而,HS的这种作用在ABA抑制剂处理的黄瓜根系中不再凸显[16]。ABA还可通过与一些硝酸盐转运蛋白相互作用来影响根的生长和结构,不同来源HA提高硝酸盐转运蛋白在不同植物中的表达水平。ABA抑制剂氟啶酮(Fld)的应用显著降低了SHA介导根生长的增加,这种效应还与Lpr的显著降低有关,表明SHA介导的根ABA浓度增加在SHA对根的生长促进作用中也很重要,这与Harris等[53]关于ABA对根系生长调节作用的研究结论一致。质膜H+-ATPase和ABA相互作用在SHA促进黄瓜生长中具有重要作用[54]。
NO和ABA信号通路存在串扰,已知NO是渗透胁迫下ABA信号通路的下游产物[49, 55],且ABA介导的NO产生可能与H 2O2有关。在SHA介导根ABA浓度增加的情况下,该过程依赖IAA和ETH信号通路,而PTIO的施用使得未经SHA处理的对照植物根ABA浓度明显增加[49, 56-57],因此ABA与SHA诱导的根NO信号的相关性仍有待深入研究。IAA和ETH信号通路均与SHA诱导的黄瓜根ABA浓度增加有关,然而抑制IAA或ETH并不能阻止SHA介导的根系生长[56-57]。同样,IAA和NO是HA对根质膜H+- ATPase活性作用的正调节因子,抑制调节因子IAA和NO不能阻止SHA介导的黄瓜根系的生长。如果SHA介导的根质膜H+-ATPase活性和根系ABA浓度的增加需要受SHA影响的几种植物调节因子(IAA、ETH和NO等)同时发挥作用,则上述研究结果可解释为SHA对根生长的促进作用包含多条信号通路传导和串扰过程[49]。NO与ETH,两种信号分子可以协同或拮抗方式相互作用。在果实成熟、脱落及叶片或花朵衰老的情况下,两种分子存在拮抗作用[49, 58]。研究证实,NO可以促进非衰老叶片组织和根中ETH浓度的增加[49, 59-60]。在某些特定情况下,作为根对铁缺乏响应的调节因子,ETH也可诱导NO生成[61]。Mora等[62]研究表明,HS介导的根ETH含量增加具有IAA信号依赖性。但由于使用了PTIO导致ETH含量增加,因此无法确定其与NO的因果关系,NO可能通过IAA依赖性信号通路影响ETH含量。
1.4 ROS信号通路及串扰信号分子ROS信号通路也可能在HS促进根系生长发育过程起到重要作用[63]。García等[64]证实堆肥HA能够在根的不同部位诱导H2O2和超氧阴离子自由基合成,其浓度适度增加时,ROS稳态相关酶活性增加,而且所有这些影响都与促进侧根发育相关。有研究表明,NO参与植物对不同类型的营养或渗透胁迫的应激,可能是由ROS信号通路介导[65]。且NO参与HA对植物生长的促进作用[46, 65-66]。NO也可触发ROS代谢,由NO诱导的蛋白S- 亚硝基化被证明是参与调节ROS代谢的蛋白酶活性的关键,如乙醇酸氧化酶(Glycollic oxidase,GO)、过氧化氢酶(Catalase,CAT)和NADPH氧化酶(NADPH oxidase,NOX)。NOX可能不参与ROS的过量产生,但在调节抗氧化剂防御、大量和微量营养元素的运输和转运中起到重要作用。已知HA具有引起ROS浓度受控增加的能力,并能有效激活维持ROS稳态主要相关酶活性,例如超氧化物歧化酶(Superoxide dismutase,SOD)、CAT和抗坏血酸氧化酶(Ascorbate oxidase,AO)[64, 67-68]。另有研究指出,从堆肥中提取的HA能够改善渗透胁迫水稻的生长[46, 64],该作用与根中ROS浓度的增加相关,且HS调节编码主要抗氧化酶及其活性的基因表达能力[64],这些结果表明堆肥HA既能诱导ROS生成又能调节ROS浓度。此外,ROS会导致IAA氧化和降解,改变植物体内IAA浓度[69],而IAA是调节ROS水平和决定ROS在氧化应激中作用的枢纽[70],因此推测ROS稳态及ROS-IAA信号通路的串扰是控制根系发育和HS介导的植物对胁迫反应的重要作用机制。虽然ROS信号通路与质膜H+-ATPase活性之间的关系尚不清楚,但研究表明,质膜H+-ATPase激活剂蓝光增加了拟南芥中ROS的产生[68],ROS信号通路与质膜H+-ATPase活性相互作用可能也参与HS促进植物根系的生长。
1.5 Ca2+信号通路及串扰信号分子Ca2+是重要的第二信使,pH值变化是植物细胞中Ca2+依赖性信号传导的原因。HS细胞受体是与H+-ATPase连接的蛋白,在受到各种类型的信号刺激后质膜电位发生变化,启动Ca2+介导的信号通路。研究证实,HS可引起水稻幼苗根细胞中胞质游离Ca2+浓度的增加[71]。HS电子供体能力与刺激H+-ATPase活性、增加胞质游离Ca2+浓度、极化依赖性Ca2+通道和膜去极化等过程相关。质膜去极化导致去极化激活Ca2+渗透通道(Depolarisation activated Ca2+ channels,DACC)开放,从而使Ca2+从质外体流向细胞质[35]。已知胞质游离Ca2+浓度的增加会刺激Ca2+与Ca2+依赖性蛋白激酶(Ca2+-dependent protein kinases,CDPK)的结合,然后质膜H+-ATPase磷酸化并与14-3-3蛋白相结合,导致质膜H+- ATPase活性增加[72]。HS作用下CDPK激活与胞质游离Ca2+浓度的增加同时发生[71]。H+是细胞电流的主要来源[10],通过膜H+通量控制细胞质pH值,调节有机和无机营养物质运输、细胞壁的膨胀和可塑性。HS处理可引起水稻幼苗侧根生长,并由质膜H+-ATPase和CDPK激活在根伸长区产生特定的H+和Ca2+通量。HA作为H+和Ca2+通量的分子诱发剂,可能作用于复杂的CDPK细胞信号级联反应的上游[71]。HA触发的信使分子包括Ca2+、ROS和游离H+[71, 73]。HA介导的根发育增加可能是由与根质膜H+- ATPase活性相关的H+和Ca2+外排协同作用的结果,而且HA诱导的根质膜H+-ATPase活性增加在HA对根生长的整合作用中起重要作用[54]。此外,关于Ca2+与其他信号分子的串扰,NO参与IAA介导侧根的发育,而Ca2+诱导侧根形成过程中IAA信号下游的NO信号[35, 74]。CDPK被IAA激活,并在根形成过程中依赖于Ca2+、钙调蛋白和NO信号分子发挥作用[35]。
质外体ROS的产生是对各种生物和非生物刺激的常见生理反应[75-76]。HS含氧官能团,主要是连接在R基团上的羧基(C(= O)OH)、羰基(—C = O)以及醇和酚中的羟基(—OH),能够调节ROS的积累和代谢能力[77-78]。在ROS-Ca2+信号串扰过程中,ROS靶向细胞膜中Ca2+通道活化的信号传导机制并参与HS调控植物根系的生长发育。通常情况下,在ROS信号传导过程中,低浓度OH·诱导Ca2+泵出,而高浓度OH·通过被动机制将Ca2+泵入[73]。ROS在Ca2+通道激活中起到非常重要的作用,包括应激调节和信号传导。研究证实,堆肥HA不仅能够调节水稻的根系发育,还能调节ROS稳态,因此,维持OH·浓度激活Ca2+通道取决于HS介导的ROS稳态机制[34]。ROS作为第二信使通过NO、ABA和Ca2+通道介导根系的发育和结构[79],因此HS介导ROS稳态机制对植物根系发育和侧根形成起到重要作用。
HS影响整个根的生长及不定侧根和根毛的增殖[4, 80],通过多条复杂的信号通路在整合路径中发挥作用。ABA不敏感型突变体的功能丧失导致侧根增殖减少[81]。ABA受体(PYL8和PYL9)作为ABA与IAA之间的串扰节点,其信号通过一组转录因子进行转导以调节侧根发育[82]。ABA不敏感转录因子的过表达也会通过减少IAA转运蛋白PINFORMED 1的表达而抑制侧根的形成[83],这种复杂的激素信号串扰可扩展至NO和ETH信号通路[84],因此IAA、NO、ABA等可以通过整合途径发挥作用,在根水平上共同调节多条信号通路。尽管HS对根系发育(侧根和不定根增殖)和结构(主根长度)的影响可以通过其对IAA、NO和ETH信号通路的影响来解释,但整个根系生长的增加(根系干物质产量)可能由与这三者无关的信号通路来调控[85],如涉及ABA信号通路及其他信号分子。
2 HS触发地上部生长发育的信号通路和串扰机制HS对根发育的作用机理研究较多,而对地上部生长作用机理的研究较少[86-87]。事实上HA通过参与根和地上部中的各种生理过程来加速地上部的生长。HA可促进根细胞中IAA和NO含量增加,且对IAA信号通路的作用调节了根质膜H+-ATPase的活性及侧根和不定根的增殖,从而促进地上部的生长[45, 63]。
2.1 ABA信号通路及串扰信号分子已有研究表明,叶面施用HS促进了黄瓜植株的地上部和根部生长,以及根系体积和主根的伸长,但减少了侧根的出现。HS可能激活了对根系结构的远距离控制,导致主要根系性状发生实质性变化。然而,叶面施用HS增加了根中IAA含量,但未增加ABA含量,而且根质膜H+-ATPase的活性并没有增加,地上部的养分也没有得到积累[88],因此推测IAA是侧根发育的重要决定因素,而ABA对整个根系的干物质积累具有潜在的重要作用[22]。此外,叶面施用HS对H+-ATPase活性的无效作用表明,只有在根水平施用HS时,H+-ATPase才是HS作用的主要靶点,并且H+-ATPase与观察到的ABA的增加无关[44]。已知HS引发了NO-IAA信号串扰,从而增加了ABA的浓度[88],调节根Lpr和水通道蛋白的活性。根ABA浓度的增加与根质膜水通道蛋白活性和根Lpr的增加有关,对于HA促进地上部生长至关重要(图 2)[74]。此外,根际高浓度HS沉积降低了根部Lpr,抑制了植物地上部的生长并阻止了根部吸收水分[22],这归因于HA在根表面细胞壁孔中的累积而产生的胶体应激[89]。ABA抑制剂(Fld)与HS联合使用可抑制根系生长及根系Lpr和地上部生长。Lpr受ABA调节,它直接影响质膜水通道蛋白活性[22],因此水通道蛋白的活性和根部对水分的吸收受根际HS浓度控制,从而影响地上部的生长。通常HS对地上部生长发育的影响在功能上归因于IAA、NO和ABA信号通路[89],这些信号通路通过相关蛋白调节质膜H+-ATPase活性,根质膜H+-ATPase活性似乎对HS介导的地上部生长也至关重要[90],由此推测相互级联的激素信号通路和质膜H+-ATPase共同调节HS对地上部生长的促进作用。CTK能够促进细胞分裂和细胞分化[91],在植物根部和地上部的生长中起到重要作用。Mora等[45]推测HA增强地上部生长的功能还可能与地上部CTK活性增加,与HA介导的根部质膜H+-ATPase活性的增加及根对硝酸盐的吸收密切相关。
|
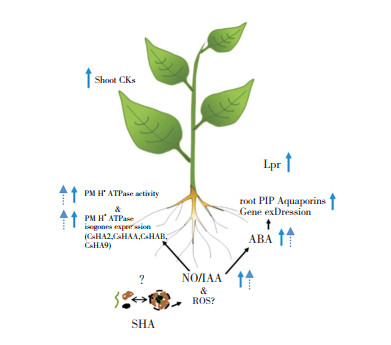
| 实线箭头表示SHA介导的模式植物(黄瓜)中促进地上部生长的 作用机理,而虚线箭头表示SHA介导的根部促生作用 Solid arrows indicate SHA-mediated mechanism of action for shoot growth promotion, while dashed arrows indicate the SHA-mediated root-promoting action in the model plant (cucumber) 图 2 SHA 对植物生长促进作用的主要途径[74] Fig. 2 Main pathways of sedimentary humic acid on plant growth-promoting effect[74] |
2.2 CTK信号通路及串扰信号分子
HS促进地上部生长相关的信号分子及其可能的串扰信号分子如表 1所示。相对于根部HS作用机制,缺乏直接的证据来证实地上部CTK和根质膜H+-ATPase活性在促进地上部生长中的因果关系。此外,HA调控根质膜H+-ATPase活性和Lpr涉及的信号通路之间的功能关系也未知。可以推测的是,SHA介导的地上部CTK浓度增加是SHA对促进地上部生长所必需的,并通过IAA- 质膜H+-ATPase相互作用调节。为了揭示SHA介导地上部CTK浓度增加对地上部生长的作用,Olaetxea等[74]将SHA和CTK信号受体抑制剂(PI-55)联合处理植物,结果表明,添加PI-55能够抑制SHA促生作用,而单独处理对植物地上部生长有促进作用,与PI-55处理相比,用SHA和PI-55处理的植物地上部CTK浓度明显增加。关于根质膜H+-ATPase活性和Lpr在SHA介导的地上部CTK浓度增加中的可能作用,质膜H+-ATPase抑制剂(DCC)的应用抑制了由SHA引起的地上部CTK的增加,而ABA抑制剂(Fld)不影响SHA介导的地上部CTK浓度增加,此外,PI-55不影响由SHA引起根IAA和ABA浓度的增加[75]。因此,根质膜H+-ATPase和CTK对于调控地上部的生长发育至关重要。
HS能够上调硝酸盐转运蛋白的表达,对玉米和小麦进行的研究表明硝酸盐增加是N转运蛋白(如NRT1.1和NRT2.1)的表达增强导致,HS对硝酸盐的吸收增加与CTK的根向地上部的流动性增强有关。研究证实,硝酸盐促进地上部生长的信号作用与促进非活性CTK形式向活性CTK形式的转化及活性CTK从根到地上部易位的增强相关[29]。此外,质膜H+-ATPase活性与基因异构体(Cs-HA2)的产生直接相关,这种异构体的上调与CTK活化形式和硝酸盐由根到地上部迁移率的增加密切相关[45, 87]。由于硝酸盐的信号作用,微量和大量矿物质营养元素从根到地上部的转移量也有所增加,这种效应与CTK作用一致[35, 87]。CTK由根到地上部的易位对硝酸盐促进地上部生长的信号作用已得到证实[92-93]。Mora等[94]研究了HS对根质膜H+-ATPase活性、硝酸盐根吸收和CTK根冠易位的影响与促进黄瓜地上部生长的潜在关系,结果表明,向黄瓜根部施用SHA会导致根质膜H+-ATPase活性和硝酸盐根冠易位增加,这与CTK根冠易位和地上部CTK浓度的增加相关。SHA介导的对CTK植物分布的作用还与地上部和根系多胺(Ployamines,PA)浓度的变化有关[45]。此外,SHA对地上部的促进作用还可能与SHA介导的根质膜H+-ATPase活性和硝酸盐根吸收增加有关。在利用油菜作为供试植物的研究中证实,根部施用SHA会影响地上部CTK信号通路中相关基因的表达[8]。实际上,施用SHA和富含CTK海藻提取物的生理效应和基因调控之间的显著相似性也证实HS对地上部的作用与CTK信号通路和功能密切相关[95]。SHA的这些作用还与根部主要养分的摄取和转运相关,这与地上部CTK的作用一致。此外,HS介导的根质膜H+- ATPase活化可能受IAA、NO和ETH信号通路的调控,而且这些信号通路也可能间接参与了HS介导地上部的促生作用[52, 61, 96-97]。Mora等[52]研究结果证实,在存在PCIB或PTIO的情况下,SHA不能促进地上部的生长,但不受ETH抑制剂〔钴(II)〕的影响,由此表明,SHA介导的地上部生长的促进作用与SHA在根中促进NO生成和IAA浓度的增加相关,这些结果与根系SHA对质膜H+ -ATPase活性和硝酸盐根冠易位的影响一致,因此,HS介导的对地上部的促生作用也依赖于NO-IAA信号串扰的调节[45, 62]。
关于HS促进地上部生长中NO和CTK信号通路间潜在的串扰机制,研究表明,两种信号分子可以协同或拮抗的方式相互作用,这取决于所研究的过程、植物生理状态和试验条件等[80]。虽然HS介导的根NO生成是否可能扩展到地上部,以及HS对根部NO、IAA和地上部CTK的介导与HS的地上部促生作用间的因果关系尚未明确揭示,但可以确定的是,HS介导的这些信号通路间可以通过HS介导的根质膜H+-ATPase激活相互级联放大联合作用。总之,HS引起的地上部促生作用涉及多个相互级联的信号通路的协同作用。Souza等[98]的研究结果支持上述结论,即植物将可溶性HS视为应激因子,触发多条信号通路及串扰过程,触发机制是激酶受体通过信号通路和转录因子的调节,通过磷酸化和下游级联来感知HS,从而启动复杂的信号级联互作。
3 HS触发根和地上部生长发育的其他信号通路植物激素及其他信号分子在HS促进根和地上部的生长发育中起到重要作用。除IAA、NO、ABA、ROS和CTK外,赤霉素(Gibberellin,GA)、SA和JA也在调节根系和地上部生长过程中起到一定的作用。ETH能够参与根毛的发育,与其他激素信号分子不同,HS作用后没有观察到类似ETH作用的根系结构变化[99],而且高浓度ETH还会抑制根系生长。由此推测,HS在促进根系和地上部生长过程中,ETH以信号串扰方式参与调控根和地上部的生长发育(表 1)。GA的作用是中断休眠、促进下胚轴伸长、花和果实发育等。HS的GA活性已被证实[100],而且HS通过与相关基因作用上调CTK和GA代谢[98],然而GA如何与其他信号通路串扰影响根和地上部生长发育还有待进一步研究。近年来科研人员还关注了HS对SA和JA信号通路的影响。已知SA和JA通常作为防御信号通路参与生物和非生物胁迫的响应。研究表明,在叶面施用HS后作为对外部信号的感知会激活SA和JA信号通路,SA和JA在根和地上部的浓度增加[74],而HS根部施用仅观察到JA在根内短期显著增加,地上部没有观察到明显变化。已知低浓度SA可增加根系生长和根系干物质产量,而JA抑制植物生长,但能够促进次生根的形成[73]。在叶面施用SHA试验中,虽然观察到JA根浓度的短期增加,但未观察到根生长的减少或侧根形成的增加,相反,观察到根干物质产量增加,次生根形成减少[74],因此这些过程可能受到参与根系发育调节的特定激素,如IAA、ABA、CTK、SA和JA等信号通路之间的比例或相对比例的调节。
4 展望由于HS异质性和超分子结构的复杂性,因此特异性地诱导特定的植物细胞受体的概率较小。HS在植物细胞中触发各种分子过程,通过促进不同分子、生化和生理过程的多条信号通路的传导和串扰,在细胞中发挥作用,然而具体的过程和机制仍需进一步研究。HS通过复杂的信号通路传导和串扰机制来调控根和地上部的生长发育。在HS促进根系生长过程中,涉及到质膜H+-ATPase活性、IAA、NO、ETH、ABA、ROS稳态和Ca2+等信号通路的传导和串扰过程。HS对地上部生长发育的调节功能与质膜H+-ATPase活性、硝酸盐、ABA和CTK信号通路的传导和串扰过程相关。HS通过复杂的代谢网络调控植物生理代谢,还可能涉及上述信号通路之外的其他信号通路的传导和串扰过程,而且多条信号通路还可能在整合路径中发挥作用。值得注意的是,质膜H+-ATPase是HS促进根系和地上部生长的重要节点,多条信号通路通过质膜H+-ATPase介导相互级联的复杂信号传导和串扰过程。
对于揭示HS促进根和地上部信号通路传导和串扰的作用机制,仍需要具体试验研究来验证相关假设,探索一些潜在的信号通路在HS介导根质膜H+-ATPase激活中的作用。目前还未明确阐明HS化学结构特征和官能团组成与植物根系主根和侧根的根长、数量和生物量,以及与地上部生长的相关性,因此HS超分子结构特征及其与根系和地上部生长相关性的研究仍有待进一步深入开展。此外,HS促生作用中防御信号和激素信号传导途径交叉重叠,相关防御信号分子(如SA和JA)与激素信号分子(IAA、GA、ABA等)的交叉、串扰、重叠和整合作用机制尚未明确阐明,仍有待进一步的研究论证。目前现有关于HS生理活性作用机制的研究都是基于对植物短期生长作用的研究,而在整个植物生长周期内的影响机制仍有待于深入研究。为了更好地揭示HS对植物促生作用影响,必须进一步深入分析生长发育过程中所涉及的信号通路的传导、交叉、串扰和整合机制,以及复杂的营养和代谢通路,为HS生理活性作用机制和农业生产应用的研究提供理论依据。
| [1] |
WYSZKOWSKI M, KORDALA N, BRODOWSKA M S. Trace element content in soils with nitrogen fertilisation and humic acids addition[J]. Agriculture, 2023, 13: 968. DOI:10.3390/agriculture13050968 |
| [2] |
RASHADA M, HAFEZA M, POPOV A. Humic substances composition and properties as anenvironmentally sustainable system: A review and way forward to soil conservation[J]. Journal of Plant Nutrition, 2022, 45(7): 1072-1122. DOI:10.1080/01904167.2021.2005801 |
| [3] |
DEHNO A H, MOHTADI A. The effect of different iron concentrations on lead accumulation in hydroponically grown Matthiola flavida Boiss[J]. Ecological Research, 2018, 33: 757-765. DOI:10.1007/s11284-018-1558-4 |
| [4] |
TIWARI J, RAMANATHAN A L, BAUDDH L, KORSTAD J. Humic substances: Structure, function and benefits for agroecosystems—a review[J]. Pedosphere, 2023, 33(2): 237-249. DOI:10.1016/j.pedsph.2022.07.008 |
| [5] |
CAO X, SCHMIDT-ROHR K. Abundant nonprotonated aromatic and oxygen-bonded carbons make humic substances distinct from biopolymers[J]. Environmental Science & Technology Letters, 2018, 5(8): 476-480. DOI:10.1021/acs.estlett.8b00107 |
| [6] |
BALTAZAR M, CORREIA S, GUINAN K J, SUJEET H N, BRAGANCA R, GONCALVES B. Recent advances in the molecular effects of biostimulants in plants: An overview[J]. Biomolecules, 2021, 11(8): 1096. DOI:10.3390/biom11081096 |
| [7] |
ELMONGY M S, WANG X Y, ZHOU H, XIA Y. Humic acid and auxins induced metabolic changes and differential gene expression during adventitious root development in azalea microshoots[J]. American Society for Horticultural Science, 2020, 55(6): 926-935. DOI:10.21273/HORTSCI14885-20 |
| [8] |
CHA J Y, KANG S H, JI M G, SHIN G I, JEONG S Y, AHN G, KIM M G, JEON J R, KIM W Y. Transcriptome changes reveal the molecular mechanisms of humic acid-induced salt stress tolerance in Arabidopsis[J]. Molecules, 2021, 26: 782. DOI:10.3390/molecules26040782 |
| [9] |
MONDA H, MCKENNA AM, FOUNTAIN R, LAMAR R T. Bioactivity of humic acids extracted from shale ore: Molecular characterization and structure-activity relationship with tomato plant yield under nutritional stress[J]. Frontiers in Plant Science, 2021, 12: 1-17. DOI:10.3389/fpls.2021.660224 |
| [10] |
AGUIAR N O, OLIVARES F L, NOVOTNY E H, CANELLAS L P. Changes in metabolic profiling of sugarcane leaves induced by endophytic diazotrophic bacteria and humic acids[J]. Peer J, 2018, 6: 1-28. DOI:10.7717/peerj.5445 |
| [11] |
CANELLAS L P, CANELLAS N O A, IRINEU L E S, OLIVARES F L, PICCOLO A. Plant chemical priming by humic acid[J]. Chemical and Biological Technologies in Agriculture, 2020, 7: 1-17. DOI:10.1186/s40538-020-00178-4 |
| [12] |
YANG F, ZHANG S S, CHENG K, ANTONIETTI M. A hydrothermal process to turn waste biomass into artificial fulvic and humic acids for soil remediation[J]. Science of the Total Environment, 2019, 686: 1140-1151. DOI:10.1016/j.scitotenv.2019.06.045 |
| [13] |
GARCIA A C, CASTRO T A T, SANTOS L A, TAVARES O C H, CASTRO R N, BERBARA R L L, GARCIA-MINA J M. Structureproperty-function relationship of humic substances in modulating the root growth of plants: A review[J]. Journal of Environmental Quality, 2019, 48: 1622-1632. DOI:10.2134/jeq2019.01.0027 |
| [14] |
VAUGHAN D, ORD B G. Uptake and Incorporation of 14C-labelled soil organic matter by roots of Pisum sativum L.[J]. Journal of Experimental Botany, 1981, 32(4): 679-687. DOI:10.1093/jxb/32.4.679 |
| [15] |
KULIKOVA N A, ABROSKIN D P, BADUN G A, CHERNYSHEVA M G, KOROBKOV V I, BEER A S, TSVETKOVA E T, SENIK S V, KLEIN O I, PERMINOVA I V. Label distribution in tissues of wheat seedlings cultivated with tritium-labeled leonardite humic acid[J]. Scientifi c Reports, 2016, 6: 1-10. DOI:10.1038/srep28869 |
| [16] |
TREVISAN S, BOTTON A, VACCARO S, VEZZARO A, QUAGGIOTTI S, NARDI S. Humic substances affect Arabidopsis physiology by altering the expression of genes involved in primary metabolism, growth and development[J]. Environmental and Experimental Botany, 2011, 74: 45-55. DOI:10.1016/j.envexpbot.2011.04.017 |
| [17] |
BERBARA R L L, GARCAI A C. Humic substances and plant defense metabolism[J]. Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment, 2014, 1: 4614-8591. DOI:10.1007/978-1-4614-8591-9_11 |
| [18] |
LIU X, ZHAO X, LYU J. Molecular characterization of sizefractionated humic acids derived from lignite and its activation of soil legacy phosphorus and Lactuca sativa growth-promoting performances[J]. ACS Omega, 2023, 8: 6838-6846. DOI:10.1021/acsomega.2c07528 |
| [19] |
YANG F, YUAN Y, LIU L, ZHANG X, GAI S, JIN Y, CHENG K. Artificial humic acid promotes growth of maize seedling under alkali conditions[J]. Environmental Pollution, 2023, 327: 1-12. DOI:10.1016/j.envpol.2023.121588 |
| [20] |
NARDI S, ERTANI A, FRANCIOSO O. Soil-root cross-talking: The role of humic substances[J]. Journal of Plant Nutrition and Soil Science, 2017, 180: 5-13. DOI:10.1002/jpln.201600348 |
| [21] |
KHAN R, KHAN M, KHAN A, SABA S, HUSSAIN F, JAN I U. Effect of humic acid on growth and crop nutrient status of wheat on two different soils[J]. Journal of Plant Nutrition, 2017, 41(1): 32-40. DOI:10.1080/01904167.2017.1385807 |
| [22] |
CASTRO T A T, BERBARA R L L, TAVARES O C H, MELLO D F G, PEREIRA E G, DE SOUZA C C B, ESPINOSA L M, GARCIA A C. Humic acids induce a eustress state via photosynthesis and nitrogen metabolism leading to a root growth improvement in rice plants[J]. Plant Physiology and Biochemistry, 2021, 162: 171-184. DOI:10.1016/j.plaphy.2021.02.043 |
| [23] |
NARDI S, SCHIAVON M, FRANCIOSO O. Chemical structure and biological activity of humic substances define their role as plant growth promoters[J]. Molecules, 2021, 26(8): 2256. DOI:10.3390/molecules26082256 |
| [24] |
ZANIN L, TOMASI N, CESCO S, VARANINI Z, PINTON R. Humic substances contribute to plant iron nutrition acting as chelators and biostimulants[J]. Frontiers in Plant Science, 2019, 10: 1-10. DOI:10.3389/fpls.2019.00675 |
| [25] |
KLECZEK A. Agricultural use of natural biostimulants-humic substances: A review[J]. Rocznik Ochrona Środowiska, 2022, 24: 1-14. DOI:10.54740/ros.2022.001 |
| [26] |
YUAN Y, TANG C, JIN Y, CHENG K, YANG F. Contribution of exogenous humic substances to phosphorus availability in soilplant ecosystem: A review[J]. Critical Reviews in Environmental Science and Technolog y, 2023, 53(10): 1085-1102. DOI:10.1080/10643389.2022.2120317 |
| [27] |
TIWARI J, RAMANATHAN A L, BAUDDH H K, KORSTAD J. Humic substances: Structure, function and benefits for agroecosystem: A review[J]. Pedosphere, 2023, 33(2): 237-249. DOI:10.1016/J.PEDSPH.2022.07.008 |
| [28] |
ROOMI S, MASI A, CONSELVAN G B, TREVISAN S, QUAGGIOTTI S, PIVATO M, ARRIGONI G, YASMIN T, CARLETTI P. Protein profifiling of Arabidopsis roots treated with humic substances: Insights into the metabolic and interactome networks[J]. Frontiers in Plant Science, 2018, 9: 1-18. DOI:10.3389/fpls.2018.01812 |
| [29] |
BONDAREVA L, KUDRYASHEVA N. Direct and indirect detoxification effects of humic substances[J]. Agronomy, 2021, 11(2): 1-13. DOI:10.3390/agronomy11020198 |
| [30] |
OLAETXEA M, MORA V, GARCIA A C, SANTOS L A, FUENTES M, GARNICA M, BERBARA R L L, ZAMARRENO A M, GARCIAMINA J M. Root-shoot signaling crosstalk involved in the shoot growth promoting action of rhizospheric humic acids[J]. Plant Signaling & Behavior, 2016, 11(4): 1-4. DOI:10.1080/15592324.2016.1161878 |
| [31] |
BEZUGLOVA O, KLIMENKO A. Application of humic substances in agricultural industry[J]. Agronomy, 2022, 12(3): 1-13. DOI:10.3390/agronomy12030584 |
| [32] |
WANG Y, LU Y, WANG L, SONG G, NI L, XU M, NIE C, LI B, BAI Y. Analysis of the molecular composition of humic substances and their effects on physiological metabolism in maize based on untargeted metabolomics[J]. Frontiers in Plant Science, 2023, 14: 1-17. DOI:10.3389/fpls.2023.1122621 |
| [33] |
ZANDONADI D B, SANTOS M P, CAIXETA L S, MARINHO E B, PERES L E P, FACANHA A R. Plant proton pumps as markers of biostimulant action[J]. Scientia Agricola, 2016, 1: 24-28. DOI:10.1590/0103-9016-2015-0076 |
| [34] |
宋鸽, POPOV A I, 石峰. 腐殖质生理活性及其与化学组成关系的研究进展[J]. 农业资源与环境学报, 2021, 38(4): 598-610. DOI:10.13254/j.jare.2020.0351 SONG G, POPOV A I, SHI F. Progress on the physiological activity ofhumic substances and correlation with its chemical composition[J]. Journal of Agricultural Resources and Environment, 2021, 38(4): 598-610. DOI:10.13254/j.jare.2020.0351 |
| [35] |
RAMOS A C, DOBBSS L B, SANTOS L A, FERNANDES M S, OLIVARES F L, AGUIAR N O, CANELLAS L P. Humic matter elicits proton and calcium fluxes and signaling dependent on Ca2+-dependent protein kinase (CDPK) at early stages of lateral plant root development[J]. Chemical and Biological Technologies in Agriculture, 2015, 2: 1-12. DOI:10.1186/s40538-014-0030-0 |
| [36] |
NASIROLESLAMI E, MOZAFARI H, SADEGHI-SHOAE M, HABIBI D, SANI B. Changes in yield, protein, minerals, and fatty acid profile of wheat (Triticum aestivum L.) under fertilizer management involving application of nitrogen, humic acid, and seaweed extract[J]. Journal of Soil Science and Plant Nutrition, 2021, 21: 2642-2651. DOI:10.1007/s42729-021-00552-7 |
| [37] |
RATHOR P, GORIM L Y, THILAKARATHNA M S. Plant physiological and molecular responses triggered by humic based biostimulants- a way forward to sustainable agriculture[J]. Plant and Soil, 2023, 492: 31-60. DOI:10.1007/s11104-023-06156-7 |
| [38] |
CONSELVAN G B, FUENTES D, MERCHANT A, PEGGION C, FRANCIOSO O, CARLETTI P. Effects of humic substances and indole-3-acetic acid on Arabidopsis sugar and amino acid metabolic profile[J]. Plant and Soil, 2018, 426: 17-32. DOI:10.1007/s11104-018-3608-7 |
| [39] |
AMPONG K, THILAKARANTHNA M S, GORIM L Y. Understanding the role of humic acids on crop performance and soil health[J]. Frontiers in Agronomy, 2022, 4: 1-14. DOI:10.3389/fagro.2022.848621 |
| [40] |
ZANDONADI D B, SANTOS M P, DOBBSS L B, OLIVARES F L, CANELLAS L P, BINZEL M L, OKOROKOVA-FACANHA A L, FACANHA A R. Nitric oxide mediates humic acids-induced root development and plasma membrane H+-ATPase activation[J]. Planta, 2010, 231: 1025-1036. DOI:10.1007/s00425-010-1106-0 |
| [41] |
TREVISAN S, PIZZEGHELLO D, RUPERTI B, FRANCIOSO O, SASSI A, PALME K, QUAGGIOTTI S, NARDI S. Humic substances induce lateral root formation and expression of the early auxinresponsive IAA19 gene and DR5 synthetic element in Arabidopsis[J]. Plant Biology, 2010, 12: 604-614. DOI:10.1111/j.1438-8677.2009.00248.x |
| [42] |
NUNES R O, DOMICIANO G A, ALVES W S, MELO A C A, NOGUEIRA F C S, CANELLAS L P, OLIVARES FL, ZINGALI R B, SOARES M R. Evaluation of the effects of humic acids on maize root architecture by label-free proteomics analysis[J]. Scientifi c Reports, 2019, 9(1): 1-11. DOI:10.1038/s41598-019-48509-2 |
| [43] |
ZANDONADI D B, SANTOS M P, DOBBSS L B, OLIVARES F L, CANELLAS L P, BINZEL M L, OKOROKOVA-FACANHA A L, FACANHA A R. Nitric oxide mediates humicacids-induced root development and plasma membrane H+-ATPase activation[J]. Planta, 2010, 231: 1025-1036. DOI:10.1007/s00425-010-1106-0 |
| [44] |
HITA D, FUENTES M, FEMANDEZ V, OLAETXEA M, GARCIAMINA J M. Discriminating the short-term action of root and foliar application of humic acids on plant growth: Emerging role of jasmonic acid[J]. Frontiers in Plant Science, 2020, 11: 1-14. DOI:10.3389/fpls.2020.00493 |
| [45] |
MORA V, BACAICOA E, ZAMARRENO A M, AGUIRRE E, GARNICA M, FUENTES M, GARCIA-MINA J M. Action of humic acid on promotion of cucumber shoot growth involves nitrate-related changes associated with the root-to-shoot distribution of cytokinins, polyamines and mineral nutrients[J]. Journal of Plant Physiology, 2010, 167: 633-642. DOI:10.1016/j.jplph.2009.11.018 |
| [46] |
JUNG H, KWON S, KIM J H, JEON J R. Which traits of humic substances are investigated to improve their agronomical value?[J]. Molecules, 2021, 26(3): 760. DOI:10.3390/molecules26030760 |
| [47] |
OLAETZEA M, MORA V, BACAICOA E, BAIGORRI R, GAMICA M, FNENTES M, EDERRA I. ABA-regulation of root hydraulic conductivity and aquaporin gene-expression is crucial to the plant shoot rise caused by rhizosphere humic acids[J]. Plant Physiology, 2015, 169: 2587-2596. DOI:10.1104/pp.15.00596 |
| [48] |
OLDROYD G E D, LEYSER O. A plant's diet, surviving in a variable nutrient environment[J]. Science, 2020, 368(6486): 1-19. DOI:10.1126/science.aba0196 |
| [49] |
CHEN W W, YANG J L, QIN C, JIN C W, MO J H, YE T, ZHENG S J, NOTES A. Nitric oxide acts downstream of auxin to trigger root ferricchelate reductase activity in response to iron deficiency in Arabidopsis[J]. Plant Physiology, 2010, 154: 810-819. DOI:10.1104/pp.110.161109 |
| [50] |
FRESCHI L. Nitric oxide and phytohormone interactions: Current status and perspectives[J]. Frontiers in Plant Science, 2013, 4: 1-22. DOI:10.3389/fpls.2013.00398 |
| [51] |
TERRILE M C, PARIS R, CALDERON-VILLALOBOS L I, IGLESIAS M J, LAMATTINA L, ESTELLE M, CASALONGUE C A. Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor[J]. The Plant Journal, 2012, 70: 492-500. DOI:10.1111/j.1365-313X.2011.04885.x |
| [52] |
MORA V, BACAICOA E, BAIGORRI R, ZAMARRENO A M, GARCIA-MINA J. NO and IAA key regulators in the shoot growth promoting action of humic acid in Cucumis sativus L.[J]. Journal of Plant Growth Regulation, 2013, 33: 430-439. DOI:10.1007/s00344-013-9394-9 |
| [53] |
HARRIS J M, ONDZIGHI-ASSOUME C A. Environmental nitrate signals through abscisic acid in the root tip[J]. Plant Signaling & Behavior, 2017, 12(1): 1-6. DOI:10.1080/15592324.2016.1273303 |
| [54] |
GARCIA A C, OLAETXEA M, SANTOS L A, MORA V, BAIGORRI R, FUENTES M, ZAMARRENO A M, BERBARA R L L, GARCIAMINA J M. Involvement of hormone-and ROS-signaling pathways in the beneficial action of humic substances on plants growing under normal and stressing conditions[J]. BioMed Research International, 2016, 1-13. DOI:10.1155/2016/3747501 |
| [55] |
HANCOCK J T, NEILL S J, WILSON I D. Nitric oxide and ABA in the control of plant function[J]. Plant Science, 2011, 181: 555-559. DOI:10.1016/j.plantsci.2011.03.017 |
| [56] |
BRIGHT J, DESIKAN R, HANCOCK J T, WEIR I S, NEILL S J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis[J]. The Plant Journal, 2006, 45: 113-122. DOI:10.1111/j.1365-313X.2005.02615.x |
| [57] |
ZHANG A, ZHANG J, ZHANG J, YE N, ZHANG H, TAN M, JIANG M. Nitric oxide mediates brassinosteroid-induced ABA biosynthesisinvolved in oxidative stress tolerance in maize leaves[J]. Plant & Cell Physiology, 2011, 52: 181-192. DOI:10.1093/pcp/pcq187 |
| [58] |
ZHU S, ZHOU J. Effect of nitric oxide on ethylene production in strawberry fruit during storage[J]. Food Chemistry, 2007, 100: 1517-1522. DOI:10.1016/j.foodchem.2005.12.022 |
| [59] |
MUR L A, MANDON J, PERSIJN S, CRISTESCU S M, MOSHKOV I E, NOVIKOVA G V, HALL M A, HARREN F J M, HEBELSTRUP K H, GUPTA K. Nitric oxide in plants: An assessment of the current state of knowledge[J]. AOB Plants, 2013, 5: 1-17. DOI:10.1093/aobpla/pls052 |
| [60] |
FISCHER A M. The complex regulation of senescence[J]. Critical Reviews in Plant Sciences, 2012, 31: 124-147. DOI:10.1080/07352689.2011.616065 |
| [61] |
GARCIA M J, SUAREZ V, ROMERA F J, ALCANTARA E, PEREZVICENTE R. A new model involving ethylene, nitric oxide and Fe to explain the regulation of Fe-acquisition genes in strategy I plants[J]. Plant Physiology and Biochemistry, 2011, 49: 537-544. DOI:10.1016/j.plaphy.2011.01.019 |
| [62] |
MORA V, BAIGORRI R, BACAICOA E, ZAMARRENNO A M, GARCIA-MINA J M. The humic acid-induced changes in the root concentration of nitric oxide, IAA and ethylene do not explain the changes in root architecture caused by humic acid in cucumber[J]. Environmental and Experimental Botany, 2012, 76: 24-32. DOI:10.1016/j.envexpbot.2011.10.001 |
| [63] |
HERNANDEZ-CAMPOS R, ROBLES C, MUNIZ-BECERA S, PEREZ-ÁLVAREZ S, LOERA-CORRAL O. Humic extract as a biostimulant in crops subjected to abiotic stress[J]. Agrociencia, 2023, 57(5): 1-18. DOI:10.47163/agrociencia.v57i5.2942 |
| [64] |
GARCIA A C, SANTOS L A, SOUZA L G A, TAVARES O C H, ZONTA E, GOMES E T M, GARCIA-MINA J M, BERBARA R L L. Vermicompost humic acids modulate the accumulation and metabolism of ROS in rice plants[J]. Journal of Plant Physiology, 2016, 192: 56-63. DOI:10.1016/j.jplph.2016.01.008 |
| [65] |
MORA V, OLAETXEA M, BACAICOA E, BAIGORRI R, FUENTES M, ZAMARRENO A M, GARCIA-MINA J M. Abiotic stress tolerance in plants: Exploring the role of nitric oxide and humic substances[M]. Berlin: Springer, 2014: 243-264.
|
| [66] |
KHAN M N, MOBIN M, MOHAMMAD F, CORPAS F J. Nitric xide in plants: Mand role in stress hysiology[M]. Berlin: Springer, 2014: 281-296.
|
| [67] |
AGUIAR N O, MEDICI L O, OLIVARES F L, DOBBSS L B, TORRES-NETTO A, SILVA S F, NOVOTNY E H, CANELLAS L P. Metabolic profle and antioxidant responses during drought stress recovery in sugarcane treated with humic acids and endophytic diazotrophic bacteria[J]. Annals of Applied Biology, 2016, 168: 203-213. DOI:10.1111/aab.12256 |
| [68] |
EL‐ESAWI M, ARTHAUT L D, JOURDAN N, HARLINGUE A D, LINK J, MARTINO C F, AHMAD M. Blue-light induced biosynthesis of ROS contributes to the signalling mechanism of Arabidopsis cryptochrome[J]. Scientific Reports, 2017, 7: 1-9. DOI:10.1038/s41598-017-13832-z |
| [69] |
彭李顺, 曹峥英, 杨本鹏, 蔡文伟. 植物激素对铝毒胁迫反应调控的研究进展[J]. 广东农业科学, 2022, 49(12): 10-19. DOI:10.16768/j.issn.1004-874X.2022.12.002 PENG L S, CAO Z Y, YANG B P, CAI W W. Research advance in the regulatory mechanism of phytohormone for plant responses to aluminum toxicity stress[J]. Guangdong Agricultural Sciences, 2022, 49(12): 10-19. DOI:10.16768/j.issn.1004-874X.2022.12.002 |
| [70] |
邢俊连, 彭歆. 水稻耐铝毒害生理和分子机制研究进展[J]. 广东农业科, 2022, 49(9): 20-30. DOI:10.16768/j.issn.1004-874X.2022.09.003. XING J L, PENG X. Research progress in physiological and molecular mechanism of aluminum tolerance in rice[J]. Guangdong Agricultural Sciences, 2022, 49(9): 20-30. DOI:10.16768/j.issn.1004-874X.2022.09.003. |
| [71] |
LAMAR R. Possible role for electron shuttling capacity in elicitation of plant biostimulant activity of humic substances on plant growth enhancement[J]. The Chemical Biology of Plant Biostimulants, 2020, 97-122. DOI:10.1002/9781119357254.ch4 |
| [72] |
MIAO R, RUSSINOVA E, RODRIGUEZ P L. Tripartite hormonal reg ulation of plasm a membr ane H+-ATPase a ctivity[J]. Trends in Plant Science, 2022, 27(6): 588-600. DOI:10.1016/j.tplants.2021.12.011 |
| [73] |
GAXIOLA R A, PALMGREN M G, SCHUMACHER K. Plant proton pumps[J]. FEBS Letters, 2007, 581(12): 2204-2214. DOI:10.1016/j.febslet.2007.03.050 |
| [74] |
OLAETXEA M, MORA V, BACAICOA E, BAIGORRI R, GARNICA M, FUENTES M, ZAMARRENO A M, SPICHAL L, GARCIA-MINA J M. Root ABA and H+-ATPase are key players in the root and shoot growth-promoting action of humic acids[J]. Plant Direct, 2019, 3: 1-12. DOI:10.1002/pld3.175 |
| [75] |
KHODADADO S, CHEGINI M A, SOLTANI A, AJAM NOROUZI H, SADEGHZADEH H S. Influence of foliar-applied humic acid and some key growth regulators on sugar beet (Beta vulgaris L.) under drought stress: Antioxidant defense system, photosynthetic characteristics and sugar yield[J]. Sugar Technology, 2020, 22(5): 765-772. DOI:10.1007/s12355-020-00839-6 |
| [76] |
SEWELAM N, KAZAN K, SCHENK P M. Global plant stress signaling: Reactive oxygen species at the cross-road[J]. Frontiers in Plant Science, 2016, 7: 1-21. DOI:10.3389/fpls.2016.00187 |
| [77] |
GARCIA A C, SANTOS L A, AMBROSIODE SOUZA L G, TAVARES O C H, ZONTA E, GOMES E T M, GARCIA-MINA J M, BERBARA R L. Vermicompost humic acids modulate the accumulation and metabolism of ROS in rice plants[J]. Journal of Plant Physiology, 2016, 192: 56-63. DOI:10.1016/j.jplph.2016.01.008 |
| [78] |
SILVA R M, CANELLAS N A, OLIVARES F L, PICCOLO A, CANELLAS L P. Humic substances trigger plant immune responses[J]. Chemical and Biological Technologies in Agriculture, 2023, 10: 1-12. DOI:10.1186/s40538-023-00468-7 |
| [79] |
TIWARI J, RAMANATHAN A L, BAUDDH K, KORSTAD J. Humic substances: Structure, function and benefits for agroecosystems - A review[J]. Pedosphere, 2023, 33(2): 237-249. DOI:10.1016/j.pedsph.2022.07.008 |
| [80] |
MACKIEWICZ-WALEC E, OLSZEWSKA M. Biostimulants in the production of forage grasses and turfgrasses[J]. Agriculture, 2023, 13(9): 1-33. DOI:10.3390/agriculture13091796 |
| [81] |
ROSE M T, PATTI A F, LITTLE K R, BROWN A L, JACKSON W R, CAVAGNARO T R. A meta-analysis and review of plant-growthresponse to humic substances: Practical implications for agriculture[J]. Advances in Agronomy, 2014, 124: 37-89. DOI:10.1016/B978-0-12-800138-7.00002-4 |
| [82] |
BRADY S M, SARKAR S F, BONETTA D, MCCOURT P. The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis[J]. The Plant Journal, 2003, 34: 67-75. DOI:10.1046/j.1365-3040.1999.00428.x |
| [83] |
XING L, ZHAO Y, GAO J, XIANG C, ZHU J K. The ABA receptor PYL9 together with PYL8 plays an important role in regulating lateral root growth[J]. Scientific Reports, 2016, 6: 1-13. DOI:10.1038/srep27177 |
| [84] |
INBAR-SHKOLNIK D, BAR-ZVI D. ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis[J]. The Plant Cell, 2010, 22: 3560-3573. DOI:10.1105/tpc.110.074641 |
| [85] |
SIMONTACCHI M, GARCIA-MATA C, BARTOLI C G, SANTAMARIA G E, LAMATTINA L. Nitric oxide as a key component in hormone-regulated processes[J]. Plant Cell Reports, 2013, 32: 853-866. DOI:10.1007/s00299-013-1434-1 |
| [86] |
SHAH Z H, REHMAN H M, AKHTAR T, ALSAMADANY H, HAMOOH B, MUJTABA T, DAUR I, ZAHRANNI Y, ALZAHRANI H A S, ALI S, YANG S H. Humic substances: Determining potential molecular regulatory processes in plants[J]. Frontiers in Plant Science, 2018, 9(263): 1-12. DOI:10.3389/fpls.2018.00263 |
| [87] |
ABOURAYYA M S, KASEEM N E, MAHMOUD T S M, RAKHA A M, EISA R A, AMIN O A. Impact of soil application with humic acid and foliar spray of milagro bio-stimulant on vegetative growth and mineral nutrient uptake of Nonpareil almond young trees under Nubaria conditions[J]. Bulletin of the National Research Centre, 2020, 44: 1-8. DOI:10.1186/s42269-020-00296-x |
| [88] |
OLAETXEA M, HITA D D, GARCIA C A, FUENTES M, BAIGORRI R, MORA V, GARNICA M, URRUTIA O, ERRO J, ZAMARRENO A M, BERBARA R L, GARCIA-MINA J M. Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root- and shoot- growth[J]. Applied Soil Ecology, 2018, 123: 521-537. DOI:10.1016/j.apsoil.2017.06.007 |
| [89] |
NARDI S, SCHIAVON M, MUSCOLO A, PIZZEGHELLO D, ERTANI A, CANELLAS L P, GARCIA-MINA J M. Editorial: Molecular characterization of humic substances and regulatory processes activated in plants[J]. Frontiers in Plant Science, 2022, 13: 1-2. DOI:10.3389/fpls.2022.851451 |
| [90] |
TAKAHASHI K, HAYASHI K I, KINOSHITA T. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis[J]. Plant Physiology, 2012, 159: 632-641. DOI:10.1104/pp.112.196428 |
| [91] |
陈可, 周新桥, 陈达刚, 郭洁, 陈平丽, 李逸翔, 刘传光, 陈友订. 复杂信号通路参与调控水稻粒重相关基因研究进展[J]. 广东农业科学, 2021, 48(10): 1-12. DOI:10.16768/j.issn.1004-874X.2021.10.001 CHEN K, ZHOU X Q, CHEN D G, GUO J, CHEN P L, LI Y X, LIU C G, CHEN Y D. Research progress of complicated signaling pathway related genes involving in rice grain weight[J]. Guangdong Agricultural Sciences, 2021, 48(10): 1-12. DOI:10.16768/j.issn.1004-874X.2021.10.001 |
| [92] |
SAKAKIBARA H, TAKEI K, HIROSE N. Interactions between nitrogen and cytokinin in the regulation of metabolism and development[J]. Trends in Plant Science, 2006, 11: 440-448. DOI:10.1016/j.tplants.2006.07.004 |
| [93] |
GARNICA M, HOUDUSSE F, ZAMARRENO A M, GARCIAMINA J M. The signal effect of nitrate supply enhances active forms of cytokinins and indole acetic content and reduces abscisic acid in wheat plants grown with ammonium[J]. Journal of Plant Physiology, 2010, 167: 1264-1272. DOI:10.1016/j.jplph.2010.04.013 |
| [94] |
MORA V, BACAICOA E, ZAMARRENO A, AGUIRRE E, GARNICA M, FUENTES M, GARCIA-MINA. Action of humic acid on promotion of cucumber shoot growth involves nitrate-related changes associated with the root-to-shoot distribution of cytokinins, polyamines and mineral nutrients[J]. Journal of Plant Physiology, 2010, 167(8): 633-642. DOI:10.1016/j.jplph.2009.11.018 |
| [95] |
BILLARD V, ETIENNE P, JANNIN L, GARNICA M, CRUZ F, GARCIA-MINA J M, YVIN J C, QURRY A. Two biostimulants derived from algae or humic acid induce similar responses in the mineral content and gene expression of winter oilseed rape (Brassica napus L.)[J]. Journal of Plant Growth Regulation, 2013, 33: 305-316. DOI:10.1007/s00344-013-9372-2 |
| [96] |
WATERS B M, LUCENA C, ROMERA F J, JESTER G G, WYNN A N, ROJAS C L, ALCANTARA E, PEREZ-VICENTE R. Ethylene involvement in the regulation of the H+-ATPase CsHA1 gene and of the new isolated ferric reductase CsFRO1 and iron transporter CsIRT1 genes in cucumber plants[J]. Plant Physiology and Biochemistry, 2007, 45: 293-301. DOI:10.1016/j.plaphy.2007.03.011 |
| [97] |
LUCENA C, WATERS B M, ROMERA F J, GARCIA M L, MORALES M, ALCANTARA E, PEREZ-VICENTE R. Ethylene could influence ferric reductase, iron transporter, and H+-ATPase gene expression by affecting FER (or FER-like) gene activity[J]. Journal of Experimental Botany, 2006, 57: 4145-4154. DOI:10.1093/jxb/erl189 |
| [98] |
SOUZA A C, OLIVARE F L, PERES L E P, PICCOLO A, CANELLAS L P. Plant hormone crosstalk mediated by humic acids[J]. Chemical and Biological Technologies in Agriculture, 2022, 9: 1-25. DOI:10.1186/S40538-022-00295-2 |
| [99] |
JINDO K, CANELLAS L P, ALBACETE A, SANTOS L F, ROCHA R L F, BAIA D C, CANELLAS N O A, GORON T L, OLIVARES F L. Interaction between humic substances and plant hormones for phosphorous acquisition[J]. Agronomy, 2020, 10(5): 1-18. DOI:10.3390/agronomy10050640 |
| [100] |
SAVY D, CANELLAS L, VINCI G, COZZOLINO V, PICCOLO A. Humic-like water-soluble lignins from giant reed (Arundo donax L.) display hormone-like activity on plant growth[J]. Journal of Plant Growth Regulation, 2017, 36: 995-1001. DOI:10.1007/s00344-017-9696-4 |
(责任编辑 陈丽娥)
 2024, Vol. 51
2024, Vol. 51